Pan-spinal infection: a case series and review of the literature
Introduction
Spinal epidural abscess (SEA) is a relatively uncommon central nervous infection, the incidence of which has doubled over the last decade (1). This is likely due to higher rate of detection secondary to higher index of suspicion and improved access to magnetic resonance imaging (MRI). Despite being uncommon, it is nevertheless an important entity as it can result in severe disability with permanent neurological deficit and long term spinal instability. Common presenting complaints of SEA include back pain, fever and neurological compromise. Spinal infection can also present as sepsis, and can be potentially life threatening if it evolves into septic shock causing multi-organ failure. The best practice for management of SEA remains unresolved. Arko et al. (2) described a more conservative approach towards SEA, with surgical intervention usually only employed when neurological deficit or spinal instability is present, while Epstein (3) advocated for surgical intervention, citing high failure rates for non-surgical management.
A rare subset of SEA is panspinal or holospinal infection which involves the entire spine from cervical to lumbar region. These patients with panspinal infection who develop neurological deterioration may be considered too unstable medically to be operated due to other systemic involvement (1). In patients who present with significant neurological deficit, recovery may be doubtful even if surgical intervention is undertaken. Performing extensive spinal surgery in patients with panspinal infection who are already medically unwell may result in significant morbidity and mortality. Thus, spinal surgeons may be reluctant to operate on patients with pan-spinal infection.
Prompted by our experience in managing several cases, we aimed to retrospectively review cases of pan-spinal infection treated at our hospital over the last 5 years, and compare our findings with those described in the literature.
Methods
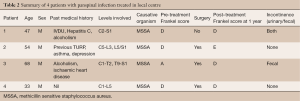
We conducted a single-centre retrospective review at The Alfred Hospital, a tertiary referral hospital located in Melbourne, Australia. Medical records over the last 5 years [2010–2014] in local institution were extracted, with final diagnoses containing keywords of “epidural abscess”, “spinal infection”, “spinal osteomyelitis”, “discitis” and “spinal septic arthritis”. Each of the records was reviewed in detail by one of the authors (CYK), and only cases in which the infection involved all 3 regions (i.e., cervical, thoracic and lumbar) were included. Of the 508 cases reviewed, 4 patients qualified based on the above criteria. Data of each case was recorded, including patient demographics, spinal levels affected, bacteriology, timing of treatment, type of surgery, relevant past medical history, pre-treatment Frankel score (Table 1) (4) as well as Frankel score 1 year post treatment.
A review of published literature on holospinal or panspinal infection was also conducted using Ovid Medline, using keyword “SEA”, “panspinal infection” or “holospinal infection”, with further cases identified in case series from the literatures. Details of the cases, including pre- and post-operative Frankel score and operative management were extracted.
Results/case illustrations
At The Alfred Hospital, we identified 4 patients with pan-spinal infection during the period 2010–2014 (Table 2).
Full table
Case 1
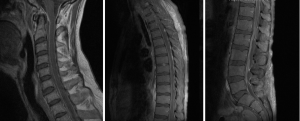
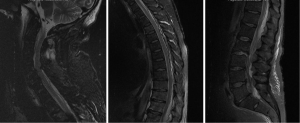
A 45-year-old male, with a past history of intravenous drug use (IVDU), chronic hepatitis C infection and alcoholism, was admitted for 2-week history of weakness and paraesthesia of bilateral lower limbs, with power of 4/5 (Medical Research Council–MRC grading) (5) as well as both urinary and fecal incontinence. Blood cultures grew Methicillin-sensitive Staphylococcus Aureus (MSSA). MRI full spine revealed extensive epidural abscess along the whole spinal canal (Figure 1). An electrocardiogram (ECG) performed on admission however showed anteroseptal ST-elevation and T-wave inversion. Troponin-I was 1.74 mcg/L (Normal range, <0.03). Transthoracic echocardiography showed anteroseptal wall hypokinesis, consistent with recent myocardial infarction (MI). Due to high perioperative MI risk, the patient was treated non-surgically with initially empirical intravenous ceftriaxone, flucloxacillin and vancomycin, and later changed to intravenous cefazolin for 6 weeks, and oral rifampicin and fusidic acid for another 3 months. On review at 1 year, power in his lower limbs bilaterally was 4+/5, maintaining Frankel grade D.
Case 2
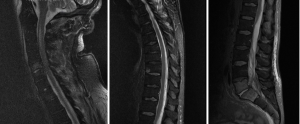
A 54-year-old male presented with 2-week history of back pain and fever. Power was 4/5 in all 4 limbs on neurological examination. Blood culture grew MSSA. MRI spine showed anterior subdural collection extending down to T7, and posterior subdural collection below T7 level extending to L3 level (Figure 2). There was also C5/6 and L5/S1 discitis, with associated epidural abscess at both levels. Due to sepsis-related organ dysfunction, he was subsequently intubated for ventilatory support, and required haemodialysis for acute renal failure. Empirical intravenous flucloxacillin, vancomycin and meropenem was commenced, with latter two discontinued once MSSA was confirmed in blood cultures. The patient was operated 27 hours post admission, in which L2-S1 laminectomy and L5/S1 discectomy was performed for associated discitis and maximal compression at that level. Durotomy was performed and washout of subdural collection was done using an infant feeding catheter. On day 2 post-operatively, the patient returned to the operating room for re-debridement of the lumbar spine. Laminectomies and washout at thoracic and cervical level were also performed. Later, C5 anterior corpectomy and fusion as well as C4–6 posterior fusion was performed 4 days following first operation. The patient eventually required L5/S1 posterior lumbar interbody fusion one month after for unstable L5/S1 joint. He continued on oral rifampicin and fusidic acid for 1 year, which were later changed to long term flucloxacillin. Review at 1 year in outpatient clinic revealed normal power in all 4 limbs and normal continence.
Case 3
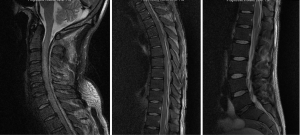
A 66-year-old male with a history of heavy alcohol use presented with 2-day history of back pain and fever, which was complicated by septic shock requiring vasopressor support. He had multiorgan failure including respiratory failure requiring intubation and ventilation, acute renal failure necessitating haemodialysis, severe hypokalemia, intestinal pseudo-obstruction, and cardiac arrhythmias. Blood culture revealed MSSA. Neurological examination (with sedation turned off for 90 minutes) revealed tetraplegia of all 4 limbs. MRI spine (Figure 3) demonstrated extensive epidural abscess throughout spinal canal. He was commenced on empirical intravenous piperacillin/tazobactam, flucloxacillin and vancomycin, which was later changed to benzylpenicillin according to sensitivity, and continued for a total of 6 weeks, This was followed by 6 months’ of oral rifampicin and fusidic acid. The patient was operated upon 64 hours post admission, with initially C2/3 anterior cervical discectomy and fusion and evacuation of subdural abscess. Durotomy was performed and a subdural washout was performed using an infant feeding catheter. C6-T1 and T12-L1 laminectomies and washout were also performed on the same day. MRI done one day after operation showed persistent collection causing cord compression. Patient subsequently had a re-do C6/7 laminectomy, and evacuation of infected epidural collection. On review one year later, patient still had lower limb weakness, but was able to ambulate with walking aids.
Case 4
A 32-year-old male, presented with 5-day history of fever, lower back pain, left shoulder pain, left lower limb pain and urinary hesitancy. Power was 4+/5 in all 4 limbs on neurological examination. Blood culture revealed MSSA. MRI spine demonstrated extensive posterior epidural abscess involving entire spine (Figure 4). He was started on empirical intravenous vancomycin and meropenem, which was later changed to flucloxacillin for a total duration of 6 weeks. Patient was operated 20 hours post admission and had C2-T2 decompressive laminectomies and non-instrumented fusion and T11-L1 laminectomies. Washout of epidural space was carried out during the same surgery using infant feeding catheter both rostrally and caudally. He continued on oral clindamycin for another five weeks prior to self cessation. He eventually required C3-T2 posterior instrumented fusion 6 months post operatively due to progressive cervical kyphosis. At 1 year post-operation, patient complained of subjective decrease of left lower limb light touch sensation, with otherwise normal neurological function.
Results and discussion
Using Ovid Medline, we identified 22 cases in 21 published English language case reports of extensive spinal infection in adults and its subsequent management and respective outcome (Table 3). Of these, 6 patients were managed without operative intervention and 16 patients had surgical intervention. All patients were managed with appropriate intravenous antibiotics.

Full table
Together with our 4 cases, we identified a total of 26 reported cases of holospinal or panspinal infection, with 19 patients treated with surgical intervention and antibiotics, whilst the other 7 were treated conservatively with antibiotics without surgery. Of the total, 15 out of 19 patients (79%) treated surgically had improvement of one or more grades based on Frankel grading system, compared with 3 out of 7 (43%) patients treated non-surgically. Benefits of neurological recovery even extends to improvement of two or more grades of Frankel score, with 32% of patients (6 out of 19) in surgical group achieving that while only 14% of patients (1 out of 7) in non-surgical group had such benefit. This is noteworthy as it suggests a greater chance of regaining significant functional capacity for patients treated surgically when compared with their non-surgical counterparts.
Morbidity and mortality attributed to acute spinal operations in these patients were not as high as one might predict, with no mortality reported in our series of four patients. Of 22 cases reported in literature, there were 3 deaths, 1 from the non-surgical group while the other 2 were from the surgical group. This overall mortality rate was 14% for non-surgical group and 13% for the surgical group. All three patients appeared to have died directly or indirectly related to the sepsis secondary to panspinal infection.
Principles for management of panspinal infection
There is no standard recommended surgery for panspinal infection, but the surgeon needs to be flexible in the approach. Indeed, panspinal infection had been considered as a relative contraindication to surgical intervention due to being “impractical to perform decompressive laminectomy along the whole spine” (5). This can however be overcome by doing several segmental laminectomies and washout caudally and rostrally using small drains, such as infant feeding catheter (1). The principles and approach for surgical interventions should be individualized and depends on the location of maximal abscess or compression. The source of infection, e.g., discitis, and surgical access to infection should also be taken into consideration. The infected disc should be excised with discectomy and durotomy should be performed if abscess or collection is located in the subdural space. Fusion may be required if there is spinal instability or progressive deformity, which may occur in cases with extensive bony or disc destruction or as a consequence of multilevel laminectomies.
This was certainly the case in our series, in which surgical interventions were performed at levels showing maximal cord compression, with the surgical approach to the collection determined by whether it was anterior or posterior. Spinal fusion procedures were performed either acutely during initial surgery or non-urgently (weeks or even months) when there were signs of spinal instability after having recovered from sepsis.
While the small number of cases in this series and review preclude conclusions being drawn on the optimal timing of surgery, it appears that surgical intervention should be advocated to be performed as soon as possible (3), as outcomes appear to correlate with the duration of neurological deficit.
Microbiology and antibiotics
All cases in our series were associated with Staphylococcus aureus, which is the most common organism isolated in epidural abscesses and associated with up to two-thirds of cases reported (2). Whilst we did not find infections associated with methicillin resistant strains, there have been reported cases associated with methicillin-resistant Staphylococcus aureus (16) and methicillin-resistant coagulase negative Staphylococcus (7). The increasing prevalence of MRSA infections in community acquired infections certainly raises further concern (27). In addition to source control where feasible, antimicrobial treatment should therefore include an anti-staphylococcal beta-lactam with vancomycin.
Limitations
Only a small number of cases have been identified at our institution and in the literature, reflecting the rarity of this condition. The association between surgical intervention and outcome observed in the cases identified may be confounded by other factors, but it is unlikely that further high quality evidence will be available to guide practice. We used the Frankel score to evaluate outcomes; while this does not quantify power grading of each muscle group, it does reflect patients’ gross functional capacity.
Conclusions
This retrospective review with literature review demonstrated that surgical intervention may be associated with an improved neurological recovery for patients with panspinal infection, in comparison with intravenous antibiotics alone. Moreover, surgical intervention did not appear to cause further associated morbidity and mortality. Whilst there is a trend to treat epidural abscess medically, from the evidence we have presented, we do not recommend this non-operative approach be used for panspinal infection, especially when associated with progressive neurological deficit. Rather, these patients should be considered for aggressive surgical intervention, if they are medically fit for surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Darouiche RO. Spinal epidural abscess. N Engl J Med 2006;355:2012-20. [Crossref] [PubMed]
- Arko L 4th, Quach E, Nguyen V, et al. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus 2014;37:E4. [Crossref] [PubMed]
- Epstein NE. Timing and prognosis of surgery for spinal epidural abscess: A review. Surg Neurol Int 2015;6:S475. [PubMed]
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969;7:179-92. [Crossref] [PubMed]
- Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty's Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain 2010;133:2838-44. [Crossref] [PubMed]
- Duc C, Grange L, Gaudin P, et al. Extensive primary epidural abscess. Report of a case. Joint Bone Spine 2002;69:312-5. [Crossref] [PubMed]
- Lin WS, Kao HW, Cheng CA. Panspinal epidural abscess concomitant with meningitis. Am J Emerg Med 2013;31:1155.e5-6. [Crossref] [PubMed]
- Manickam A, Marshman LA, Korah I. Pan-regional (cervico-thoraco-lumbo-sacral) spinal epidural abscess with multi-level discitis, vertebral body osteomyelitis and facet joint septic arthritis: Complete resolution with non-operative management. Interdisciplinary Neurosurgery: Advanced Techniques and Case Management 2014;1.
- O'Brien C, Lenehan B, Street J. Non-operative management of an extensive anteriorly located epidural abscess. J Clin Neurosci 2011;18:1401-2. [Crossref] [PubMed]
- Simpson RK Jr, Azordegan PA, Sirbasku DM, et al. Rapid onset of quadriplegia from a panspinal epidural abscess. Spine (Phila Pa 1976) 1991;16:1002-5. [Crossref] [PubMed]
- Van Bergen J, Plazier M, Baets J, et al. An extensive spinal epidural abscess successfully treated conservatively. J Neurol Neurosurg Psychiatry 2009;80:351-3. [Crossref] [PubMed]
- Ansari A, Davies DW, Lohn JW, et al. Extensive spinal epidural abscess associated with an unremarkable recovery. Anaesth Intensive Care 2004;32:825-9. [PubMed]
- Burton KR, Wang X, Dhanoa D. Holocord spinal epidural abscess in a pregnant patient presenting as premature labour: a rare presentation of an unusual diagnosis. CJEM 2014;16:334-8. [Crossref] [PubMed]
- Elsamaloty H, Elzawawi M, Abduljabar A. A rare case of extensive spinal epidural abscess in a diabetic patient. Spine (Phila Pa 1976) 2010;35:E53-6. [Crossref] [PubMed]
- ERDEM Y, KARATAY M, ÇELİK H, et al. Minimally Invasive Surgery of Extensive Spinal Epidural Abscess: A Case Report. J Neurol Sci 2014;31:641-5.
- Gorchynski J, Hwang J, McLaughlin T. A methicillin-resistant Staphylococcus aureus-positive holospinal epidural abscess. Am J Emerg Med 2009;27:514.e7-9. [Crossref] [PubMed]
- Hwang DW, Lee CW, Nam HT, et al. Long level (t4-l1) spinal epidural abscess in a diabetic patient - a case report -. Asian Spine J 2008;2:55-8. [Crossref] [PubMed]
- Jackson F, Assam S. Extensive spinal epidural abscess treated by laminectomy and hypothermia. Case report. J Neurosurg 1964;21:237-9. [Crossref] [PubMed]
- Lau D, Maa J, Mummaneni PV, et al. Holospinal epidural abscess. J Clin Neurosci 2014;21:517-20. [Crossref] [PubMed]
- Panagiotopoulos V, Konstantinou D, Solomou E, et al. Extended cervicolumbar spinal epidural abscess associated with paraparesis successfully decompressed using a minimally invasive technique. Spine (Phila Pa 1976) 2004;29:E300-3. [Crossref] [PubMed]
- Riaz S, Mahmood JK. Extensive spinal epidural abscess. J Ayub Med Coll Abbottabad 2007;19:64-7. [PubMed]
- Shoakazemi A, Amit A, Nooralam N, et al. Panspinal epidural and psoas abscess with secondary cervical disc space infection. Ulster Med J 2013;82:23-5. [PubMed]
- Smith C, Kavar B. Extensive spinal epidural abscess as a complication of Crohn's disease. J Clin Neurosci 2010;17:144-6. [Crossref] [PubMed]
- Tahir MZ, Hassan RU, Enam SA. Management of an extensive spinal epidural abscess from C-1 to the sacrum. Case report. J Neurosurg Spine 2010;13:780-3. [Crossref] [PubMed]
- Talwalkar N, Debnath U, Medhian S. Quadriparesis from a panspinal extradural abscess following pneumococcal meningitis. Acta Orthop Belg 2006;72:647-50. [PubMed]
- Urrutia J, Rojas C. Extensive epidural abscess with surgical treatment and long term follow up. Spine J 2007;7:708-11. [Crossref] [PubMed]
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010;23:616-87. [Crossref] [PubMed]



