Anterior to psoas (ATP) fusion of the lumbar spine: evolution of a technique facilitated by changes in equipment
Introduction
In spinal fusion, lateral interbody cages have biomechanical advantages over other interbody cages. They have the largest surface area for endplate support and graft retention, spanning both lateral cortical rims while sparing the Anterior Longitudinal Ligament (ALL) they restore and maintain disc height while adding stability even before supplemental fixation (1). The standard approach for their insertion is by a 90 degrees lateral transpsoas method (2). This is a relatively bloodless technique (50 mLs per level) compared to other interbody fusion techniques but has its limitations (3). Neuro-monitoring is essential and the L4/5 level can be difficult because of iliac crest obstruction or an anterior plexus position (4-6). Sensory disturbances and thigh weakness are common complications (7-10). An oblique approach, with the patient in a lateral position passes anterior to the iliac crest, remains retroperitoneal, and eliminates the need for neuro-monitoring by staying entirely anterior to psoas. Reports of this approach are few, most obviously beginning with Mayer in 1997 and more recently Silvestre in 2012 (2,11), a similar approach called OLIF 25TM has recently been published by Medtronic Inc (Memphis, TN, USA). While the oblique approach to the disc spaces appears logical, an oblique direction during insertion of the interbody means that short iliac crest graft or TLIF style cages have been recommended to avoid contralateral neural injury, should the graft go too far. The problem was thus how to combine an oblique approach with use of larger lateral type cages, the solution required developing special instruments.
To gain 90 degrees lateral access to the L4/5 disc without going through the bone required offsetting the instruments to pass under the iliac crest, combined with offsetting retractors to retract the psoas in a controlled fashion. This was achieved and allows placement of large lateral interbody cages in the same trajectory as achieved by a typical transpsoas approach. We report the surgical technique and the early outcomes in the first 21 consecutive cases.
Methods
Surgeries for interbody spinal fusion via ATP approach were performed in 21 consecutive patients by the senior author (KS), between November 2011 and February 2013.
Mean age at the time of surgery was 62.4±7.4 years. There were 13 males and 8 females in this group (ratio 1.6:1).
There was a 6 months minimum clinical follow up, with imaging to assess fusion at 6 and 12 months.
Thirty-two levels were instrumented with ATP technique and included 2 L5/S1 levels (3 other patients that required fusion at L5/S1 had respectively an ALIF (1 patient) and PLIF (2 patients).
Indications included symptomatic degenerative lumbar spondylosis with spondylolisthesis in 4 cases. Eleven patients had previous lumbar spine operations including 4 patients who had previous instrumented lumbar spinal fusion suffering an adjacent level disease and 3 who had decompressive surgery and placement of a dynamic stabilization device.
All patients were assessed with the Oswestry Disability Index (ODI), Visual Analog Scale 100 mm for back pain (VASb) and for leg pain (VASl) preoperatively, at 3, 6 and 12 months. One patient did not complete the preoperative Questionnaires and we only report their scores at follow-up and their pattern of clinical improvement or worsening.
No patients were lost at follow-up. Last follow-up was at 12 months for 9 patients and the rest had 6 months follow up.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics [version 21, IBM corporation and other(s)] software. Normal distribution of the sampling for the different scores in the pre-operative period and at 3, 6 and 12 months after surgery were verified using the Kolmogorov-Smirnov test. Data was analyzed using the one-way within-groups analysis of variance (ANOVA) and simple planned contrast analysis was performed in order to determine the impact of surgery based on ODI, VASb and VASl scale scores in the pre-operative period and 3, 6, 12 months after surgery, respectively. Differences were determined to be significant with a P value less than 0.05.
Surgical technique
The most obvious use for this approach is access to L4/5 disc space so this level will be described. We suggest a headlight and loupes, with illuminated retractor blades, if available. Mayer suggested a microscope (12). A video of the procedure has been published recently (13).
Patient positioning
This is similar to positioning and taping for a transpsoas approach. The patient is placed in lateral decubitus. The hip is positioned just below the table break and is gently flexed to relax the psoas muscle and femoral nerve. A pillow is placed in between the knees and the taping of the lower pelvis and uppermost hip and femur is performed to stabilize the spine and allow gentle traction of the pelvis on breaking the table. A slight break can be helpful to stretch the skin and open a collapsed disc space although is not routinely performed. AP fluoroscopy is used to ensure there is no rotation of the spine before the chest is then taped and the lateral fluoroscopy is done to ensure that the target disc space is perpendicular to the floor, adjusting the table as required. The skin is then marked overlying the disc spaces of interest and showing the anterior projection of the disc space (Figure 1).
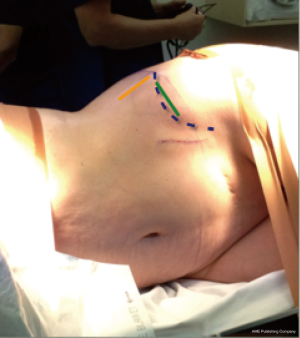
The approach is generally left side up as this reduces the need for any venous retraction although a scoliotic spine concave on the right may require a right sided approach for multilevel surgery.
Incision
In ATP cases, left side up, the surgical corridor approaches the disc space through the natural space between psoas and the common iliac vessels. The skin incision should be 1/3 below and 2/3 above the anterior projection of the disc onto the skin (Figure 1) sloping obliquely in the line of the external oblique fibers. For L4/5 this is usually about 30 mm in front of anterior superior iliac spine towards the umbilicus. A single or double level operation can comfortably be done within a 60−80 mm skin incision. Three or four levels require a longer skin incision although in scoliosis cases, access on the concave side requires only a little more. For more than 2 levels the author usually splits the deeper 2 muscles twice, having extended the external oblique split. The term “sliding window” has been used to describe the mobility of the skin incision (11).
Exposure of the disc
Following the skin incision, the external oblique fascia is cut in line of its muscle for about 50 mm and the 3 abdominal muscles are bluntly dissected in the line of their fibers. Iliohypogastric or ilioinguinal nerves may be encountered beneath the internal oblique muscle and are mobilized. The Transversalis fascia is opened as laterally as possible to avoid the peritoneum. The more yellow retroperitoneal fat is then “paddled” backwards using a pair of swabs on sticks. Dissection proceeds initially postero-laterally and then at about an angle of 20 degrees off vertical, pushing the retroperitoneal fat (with peritoneum and ureter) antero-medially, until psoas muscle is seen almost immediately. Psoas is an obvious bulky structure and may have a psoas minor tendon on the surface. The muscles of the lateral abdominal and pelvic walls should not be stripped clean because of the cutaneous nerves travelling in the retroperitoneal space on the abdominal wall. Psoas is gently retracted posteriorly with a handheld retractor and followed carefully around its anterior surface to reach the spine. The genitofemoral on its anterior surface nerve will be seen and retracted with psoas.
Establishing a stable exposure is very helpful. Medially, this will require retraction of retroperitoneal fat, peritoneum with ureter on its surface and on the spine, the great vessels.
We use a single L shaped blade CurvyTM (Relax Retractors, Sydney, Australia), medially with its back retracting the retroperitoneal fat while protecting the vessels and its orthogonal blade pushing psoas posteriorly. A separate straight retractor is used to retract psoas (Figure 2). A long smooth ended dissector and a Yankeur suction tip are useful to dissect the loose fatty connective tissue between psoas and the fat over the vessels, to reveal the spine, the disc space, the sympathetic chain and the segmental vessels. Some small vessels can be bipolared. Below the L4/5 disc the ileo-lumbar vein may be seen. With magnification one can see the vertical fibers of the Anterior Longitudinal Ligament (ALL).
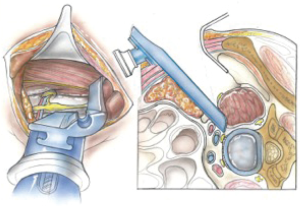
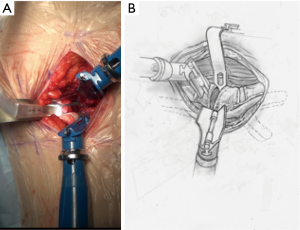
Once the disc level is confirmed by feel or X-ray, the CurvyTM retractor is positioned medially over the disc space with the “leg” over the disc and the “foot” lying in a medial-lateral direction, protecting the iliolumbar vein inferiorly (Figure 3). The curvy fixation screw is inserted into the target disc for temporary fixation. The disc is then incised with a knife to about 20–25 mm behind the ALL (Figures 4,5). A limited discectomy is performed and the reverse tooth of the selected G Clamp blade is placed beneath the lateral uncut annulus. The blade and the clamp retracting psoas are then compressed to retract psoas (Figure 6). Psoas attachments to the disc margin are separated with a dissector or Cobb elevator. The extent of the cut in the annulus limits the posterior extent of psoas retraction, which provides an end point for retraction to avoid compression of the lumbar plexus. The sympathetic chain is mobilized usually medially by dividing its tiny branches, the rami communicans. Having identified the endplates, the CurvyTM blade is now repositioned lateral to the sympathetic trunk and its screw inserted into the L5 superior endplate or body to secure the blade (Figure 6). The medial blade usually lies close to the posterior edge of the ALL.
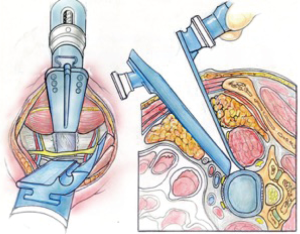
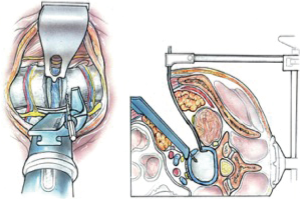
A lateral X-ray is then taken to verify the limit of the posterior retraction about mid body is adequate for disc preparation; this can be adjusted if required. The Curvy and G clamp are stable without table mounting.
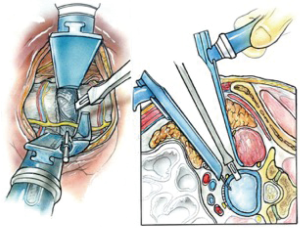
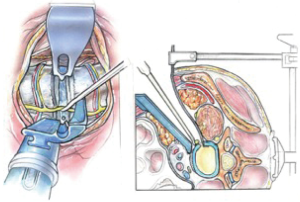
Only a limited amount of psoas retraction is needed for thorough disc preparation and contralateral release. If the retroperitoneal fat comes into the field superiorly, an abdominal pack is used or a straight Curvy™ blade is fixed to the vertebral body (Figure 7).
The iliac vessels, if seen are protected under the medial retractor blade. The ileo-lumbar vein at L5 usually sits mid-body and can be ligated between vessel clips if on occasion it overlies the disc space, or in order to retract the common iliac vein to expose the L5/1 disc or a transitional L4/5 level. It is noteworthy that the lateral position and oblique approach reveal a greater length of the iliolumbar vein making ligation easier than during supine L4/5 ALIF surgery.
Discectomy and endplate preparation
Osteophytes, if obstructing can be removed by means of rongeur or bone nibbler.
Initial discectomy with large pituitary rongeurs and disc curettes is performed. A “Dingo” Cobb elevator is placed in the disc space and impacted through the contralateral annulus and bridging osteophytes under AP X-ray control. The offset Dingo design (Figure 8) allows for a true lateral orthogonal axis for the working end of the Cobb despite the oblique access.
After completion of the discectomy and with disc distraction, the oblique trajectory also allows for direct visualization of the thecal sac and contralateral foramen to allow decompression under direct vision (14), a microscope is preferred for this.
Cage and plate insertion
Dingo design implant inserters allow for a standard lateral cage to be inserted across the disc space thus gaining bilateral cortical endplate coverage. As with conventional lateral transpsoas cages, this cage type and position is an effective method for correction of coronal deformity, lateral listhesis, restoration of foraminal height, and some correction of spondylolisthesis (1).
In selected cases with good bone density, if lordosis appears adequate and foraminal height restoration is satisfactory after cage alone, then we have supplemented the cage with a 4 hole anterior plate placed through the same window, and avoided any posterior fixation (15,16) (Figure 9).
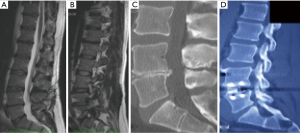
For two levels surgery, the second level can be accessed through the same incision, repeating the above procedure (Figure 10). For lumbar levels up to L2/3, a second superior muscle split may be made in the upper part of the incision, again, in the line of the external oblique muscle fibers. L5/S1 is also accessible using this approach but is more complex not least because this level always requires some vascular dissection and mobilization and usually ligation of ileolumbar vein.
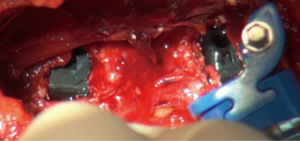
Closure is by suture approximation of muscle layers and repair of external oblique fascia.
Results
ODI, VASb and VASl scores in the immediate pre-operative period and 3, 6, 12 months after surgery are summarized in Table 1. One-way within-groups ANOVA analysis showed statistically significant differences ODI {F [3,24] =8.70, P<0.001}, VASb {F [3,18] =10.67, P<0.001} and the VASl {F [3,21] =9.67, P=0.017} scores. Simple planned contrast analysis confirmed ODI, VASb and VASl scores improvement 3, 6 and 12 months after surgery, respectively (Table 1).
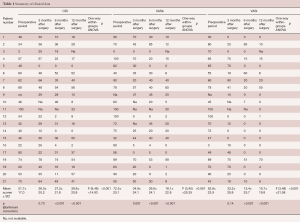
Full table
Preoperative ODI scores ranged between 2 and 80 (average 48.7). Seventeen patients had improvement of their ODI scores at last follow-up of 28 points on average (range, 4–52). Two patients had unchanged scores at their last follow-up and 2 patients had worsening of their scores (4 and 16 points respectively).
Preoperative VASb scores ranged between 0 and 100 mm (average 68 mm). Eighteen patients improved at their last follow-up 49.7 points on average (range, 2–100). One patient was unchanged (no back pain at presentation) and 1 patient had a worsening of 7 points.
Preoperative VASl scores ranged between 3 and 100 mm (average 62 mm). Nineteen patients improved their scores at follow-up between 3 and 100 mm (average 52 mm). One patient was unchanged.
Eight patients had complications related to surgery which were still present at last follow-up. These included 2 patients with weakness of hip flexion and 1 patient who had EHL weakness. Two patients had lateral cutaneous nerve (LCN) palsy on the side of the approach at their last follow-up. One patient had sympathectomy effect related to the mobilization of the sympathetic trunk. One patient, who also suffered from multiple sclerosis, experienced psoas abscess 3 months post op, after being restarted on immunosuppressant’s. The abscess was successfully treated with CT guided drainage and antibiotics alone and went on to have a good outcome.
The one patient who had a worsening in VASl scores had developed new sacro-iliac joint type symptoms thought to be an ‘adjacent segment’ biomechanical consequence of the lumbar fusion.
Discussion
Introduced in 2006, the placement of large lateral cages that span the cortical rims have been shown to provide increased stability to the segment, and restore disc height easily with often dramatic effects on coronal alignment (1,17). Until now these cages have been inserted by a transpsoas approach which requires neuro-monitoring and can be difficult at L4/5 particularly in men with a large psoas and a high iliac crest (11). In order to reduce sensory complications and difficulties particularly at L4/5, we modified Mayer’s 11 approach late in 2011 helped by the development of a custom retractor system and offset instruments to avoid the iliac crest. Initially only straight inserters were available but the use of angled (Developed for Dr. Tannoury, personal communication) inserters followed by our own Dingo inserters, led to improved cage alignment. Silvestre’s report in 2012 described 179 patients operated between 2006 and 2009 by senior author Pierre Rousoully (2). They coined the term OLIF to describe their Oblique Lumbar Interbody Fusion, used a banana shaped TLIF style cage and recommended <30 mm cages to avoid injury to the contralateral traversing nerve root. The Medtronic OLIF25TM technique described later in 2012 can use lateral cages without any fixed psoas retraction. It described insertion of cages with straight instruments into the disc space and then rotating the cage from the oblique angle as orthogonal as possible. Our early experience followed this technique although it regularly led to oblique cage positioning. It was the concern about potential contralateral nerve injury with straight instruments that led to the design of the Dingo instruments, allowing lateral cages to be consistently placed 90 degrees to the disc space.
As no clinical results are yet reported for the Medtronic technique, comparisons are so far anecdotal.
Retractors
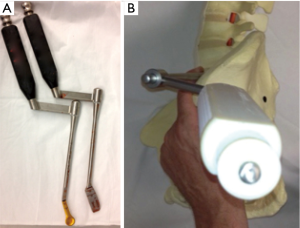
In regards to retractors, all authors have used bone fixed retractor in some form. Mayer described a frame supporting 4 blades secured to the spine with anchoring screws through the cranial and caudal blades (1). He also noted that a table based system such as Synframe (Synframe; Synthes Oberdorf, Switzerland) could be used and this has also been used by Dr Tannoury (personal communication). Silvestre used 4 Steinman pins (2). The Medtronic technique uses their MAST QuadrantTM lateral retractor system pinning cranial and caudal blades. Our technique uses one bone fixed blade anteriorly and a novel self-retaining posterior retractor.
Neuromonitoring
None of the other authors (2,12) employing an anterior to psoas approach have used neuromonitoring in routine cases although this is described in the Medtronic technique. The muscle retraction both superficial and deep, is likely to be easier with the use of muscle relaxants which would otherwise need to be reversed. This is due to the fact that the approach related neural complications are peripheral sensory or autonomic, but neither lend themselves to monitoring. The authors have no experience with monitoring cremaster muscle to detect Genitofemoral nerve problems. Furthermore, Neuromonitoring contracts the abdominal wall muscles are tight potentially hindering the approach and being counterproductive for little benefit.
Psoas retraction
A theoretical concern with the anterior to psoas approach is that the lumbar plexus may be stretched or compressed within psoas against the Transverse processes. To avoid this, we limited the psoas retraction at L4/5 to mid-body, and released psoas retraction during surgical delays.
If a more posterior position of the cage is desired, the annulus can be cut further posteriorly immediately prior to trial or cage placement and either more psoas retraction employed with G Clamp or an oblique insertion with posterior rotation technique employed (without G Clamp), letting the implant itself retract psoas briefly during the move from oblique to right angles to the spine.
Perhaps the greatest concern with this retroperitoneal approach are potential vascular injuries. From the left however, the anatomy is favorable as there is a natural corridor, well seen on magnetic resonance imaging (MRI), between psoas and the vessels and typically no vascular dissection is required. However, surgeons should be prepared and able to divide the iliolumbar vein as rarely this may overlie the L4/5 disc.
The mini open approach described here allows for direct visualization of all critical intra-abdominal structures. Any venous bleeding from ascending lumbar veins inside psoas or segmental on the vertebral body is dealt with under direct vision, which may improve control versus that achievable from within a tubular retractor. There is usually no need to ligate segmental vessels as these run at the mid-vertebral body level.
In comparing the ATP approach to ALIF at L4/5, the absence of vascular dissection and vascular retraction is an advantage particularly in elderly patients, especially as it is rarely necessary to take the ileo-lumbar veins. On the other hand, access to the L5/S1 disc going anterior to psoas and lateral to the vessels appears to require a similar degree of vascular expertise as for an L4/5 ALIF.
The sympathetic trunk has to be mobilized and our experience demonstrates that this tolerates mobilization or compression by smooth retractor blades, even sacrifice produces only warming of the affected leg that is usually unnoticed by the patient.
The genito-femoral nerve is the sensory nerve most at risk, being on the psoas and immediately under the psoas retractor blade. Injury can result in unpleasant groin neuralgia. Careful placement of the G Clamp retractor blade, limiting duration, and retractor stability are recommended to reduce potential nerve injuries.
Conclusions
The left sided anterior to psoas approach offers the most natural corridor to the disc space. The novel instruments and method described here allows for the insertion of large lateral cages between L2 and L5, without the problems associated with the transpsoas approach, particularly at L4/5.
Acknowledgements
We acknowledge Dr. David Bervini for his help with the statistical analysis.
Footnote
Conflicts of Interest: Dr. Seex holds a Patent to some of the retractors utilized in this technique. The other author has no conflicts of interest to declare.
Ethical Statement: Data were collected and analyzed after Ethics Committee approval.
References
- Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22:691-9; discussion 700. [Crossref] [PubMed]
- Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J 2012;6:89-97. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012;2012:246989.
- Berjano P, Lamartina C. Minimally invasive lateral transpsoas approach with advanced neurophysiologic monitoring for lumbar interbody fusion. Eur Spine J 2011;20:1584-6. [Crossref] [PubMed]
- Guérin P, Obeid I, Gille O, et al. Safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: a morphometric study. Surg Radiol Anat 2011;33:665-71. [Crossref] [PubMed]
- Kepler CK, Sharma AK, Huang RC. Lateral transpsoas interbody fusion (LTIF) with plate fixation and unilateral pedicle screws: a preliminary report. J Spinal Disord Tech 2011;24:363-7. [Crossref] [PubMed]
- Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 2011;15:11-8. [Crossref] [PubMed]
- Dakwar E, Le TV, Baaj AA, et al. Abdominal wall paresis as a complication of minimally invasive lateral transpsoas interbody fusion. Neurosurg Focus 2011;31:E18. [Crossref] [PubMed]
- Davis TT, Bae HW, Mok JM, et al. Lumbar plexus anatomy within the psoas muscle: implications for the transpsoas lateral approach to the L4-L5 disc. J Bone Joint Surg Am 2011;93:1482-7. [Crossref] [PubMed]
- Sharma AK, Kepler CK, Girardi FP, et al. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech 2011;24:242-50. [Crossref] [PubMed]
- Sofianos DA, Briseño MR, Abrams J, et al. Complications of the lateral transpsoas approach for lumbar interbody arthrodesis: a case series and literature review. Clin Orthop Relat Res 2012;470:1621-32. [Crossref] [PubMed]
- Moller DJ, Slimack NP, Acosta FL Jr, et al. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg Focus 2011;31:E4. [Crossref] [PubMed]
- Gragnaniello C, Seex KA. Anterior to psoas fusion of the lumbar spine. Neurosurg Focus 2013;35:Video 13.
- Le TV, Smith DA, Greenberg MS, et al. Complications of lateral plating in the minimally invasive lateral transpsoas approach. J Neurosurg Spine 2012;16:302-7. [Crossref] [PubMed]
- Elowitz EH, Yanni DS, Chwajol M, et al. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg 2011;54:201-6. [Crossref] [PubMed]
- Le TV, Vivas AC, Dakwar E, et al. The effect of the retroperitoneal transpsoas minimally invasive lateral interbody fusion on segmental and regional lumbar lordosis. ScientificWorldJournal 2012;2012:516706.
- Acosta FL, Liu J, Slimack N, et al. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. J Neurosurg Spine 2011;15:92-6. [Crossref] [PubMed]
Contributions: (I) Conception and design: All authors; (II) Administrative support: All authors; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.


