Consideration of proper operative route for interlaminar approach for percutaneous endoscopic lumbar discectomy
Introduction
Percutaneous endoscopic lumbar discectomy (PELD) is one of the most sophisticated operative procedures for the treatment of lumbar disc herniation (LDH) (1-3). The operative outcome has been already reported by several investigators and the results are comparable with those of conventional operative procedure such as open discectomy (4-9). However, PELD has an anatomical limitation for endoscope insertion, and there are three different operative approaches: interlaminar, transforaminal, and posterolateral.
The endoscope insertion for transforaminal and posterolateral approaches is a blind procedure, but it can be relatively safely performed via the “Kambin’s triangular working zone” (10). On the other hand, the insertion for interlaminar approach (ILA) is performed under endoscopic visualization, but it is impossible to completely avoid direct retraction of the nerve root and/or dural sac by operative instruments. From previous experience with open, microscopic, or microendoscopic discectomy, we recognize that some extent of transient retraction of the nerve root is acceptable. Nevertheless, we have to minimize the retraction as much as possible.
Furthermore, ILA has two different operative routes: via the shoulder of the corresponding nerve root and via the axilla of the corresponding nerve root and dural sac (1,11,12). We do not have definitive criteria for selection of an appropriate operative route for ILA. We, therefore, comparatively analyzed cases that involved these two different operative routes and have provided useful information for selecting an appropriate operative route for ILA.
Methods
Forty-one consecutive patients with LDH underwent ILA of PELD using a 7-mm diameter spinal full-endoscopic system (Richard Wolf GmbH, Knittlingen, Germany) between April 2014 and February 2016. All patients had lateral radiculopathy resistant to medical treatment, epidural steroids, and/or nerve block. To clarify the surgical benefit of ILA of PELD, we excluded patients who previously underwent discectomy at the same vertebral level. We also excluded patients with spinal canal stenosis, which required at least partial laminectomy or facetectomy.
All patients underwent ILA of PELD at only one vertebral level. Neurological examination, preoperative computed tomography (CT), and magnetic resonance imaging (MRI) were used to identify the location and type of LDH. The width of the interlaminar space was calculated on axial CT. The width was determined by the widest distance between the bilateral facet joints at the corresponding disc level (Figure 1A-C). The LDH size was evaluated by the protruded height against the antero-posterior diameter of the spinal canal (Figure 1D). We named the ratio AP size-ratio and defined an AP size-ratio >0.5 as large LDH. LDH was classified into four types according to the direction of herniation on axial MRI: shoulder, ventral, axilla, and central type (Figure 1E-H), defined as vertebral disc herniation to the outside of the dural sac (shoulder), to the ventral side of the nerve root (ventral), to the space between the nerve root and dural sac (axilla), and widely to the ventral side of the dural sac (central).

Patients were followed up for an average of 9.2 months (range, 2–24 months) postoperatively. Pre- and postoperative neurological statuses were evaluated using the modified Japanese Orthopedic Association (mJOA). Recovery rate was calculated as follows: recovery rate = postoperative mJOA—preoperative mJOA/23 (full score)—preoperative JOA score ×100. Corresponding leg pain was also evaluated using the numerical rating scale (NRS) score. Statistical analysis was performed using Students’ t-test. P values less than 0.05 were considered statistically significant.
Surgical technique
The patients were carefully log-rolled into the prone position. To enlarge the interlaminar space, a pillow was placed between the operating table and anterior iliac crest. Except in the initial two cases (epidural anesthesia), the operations were performed under general anesthesia combined with motor evoked potential (MEP) monitoring to avoid intraoperative discomfort and postoperative piriformis syndrome (13). During the operation, a fluoroscope was placed across the center of the operating table to ensure appropriate positioning. A 8-mm skin and fascia incision was made at the target spinal level under fluoroscopic guidance. The muscle attached to the underneath ligamentum flavum and inner surface of the facet joint was carefully detached using an obturator in a similar manner to the operating technique for MED (14). Next, a 30-degree angled-working sheath was inserted onto the ligamentum flavum, and the ligamentum was removed using several types of forceps. For the shoulder approach, the ligamentum was removed more toward the cranial and lateral area. After exposing the protruded intervertebral disc at the shoulder area of the corresponding nerve root, the nerve root was medially retracted using a Penfield dissector or working sheath, and the herniated nucleus or the annular tear was identified. For the axilla approach, the ligamentum was removed more toward the caudal and medial areas. Generally, the axilla area (between the nerve root and dural sac) covered with epidural fat could be observed directly under the removed ligamentum. After removal of the fat tissue and enlargement of the space using a Penfield dissector, the herniated nucleus or the annular tear was directly identified. In both operative routes, the degenerated nucleus was then removed using forceps, and the evacuated cavities were electrocoagulated by a bipolar radio-frequency electrode system (Elliquence, Baldwin, NY, USA). The extent of decompression was also confirmed with fluoroscopy using a flexible tip of the electrode system. After decompression, the working sheath was carefully removed, and skin was closed by a single suture.
Results
Forty-one patients were registered for this study; 33 patients underwent ILA via the shoulder and eight patients underwent ILA via the axilla. The mean age was 40.0 years (range, 18–59 years) and 47.9 years (range, 21–67 years) for the two sets of patients, respectively. The most affected vertebral level was L5/S1 (28 cases and 5 cases, respectively), followed by L4/5 (5 cases and 2 cases, respectively). Each location of LDH predicted by preoperative MRI is indicated in Table 1. Especially for large LDH, definitive determination of the location is relatively difficult and we had to select the ventral type of ILA. There was no significant difference between the two groups regarding the AP size-ratio, width of interlaminar space, postoperative removed disc volume, operation time, postoperative hospital stay, and blood loss (Table 1).
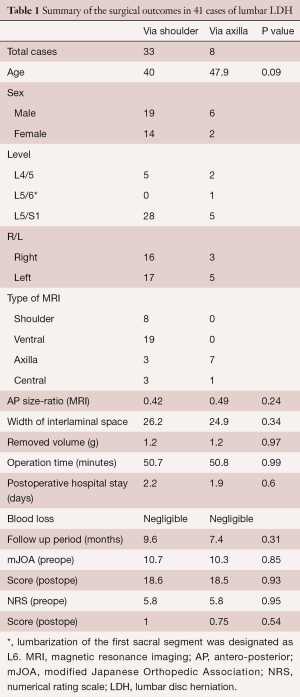
Full table
The mJOA score of both groups improved significantly from 10.7±4.8 and 10.3±4.6 to 18.6±3.3 and 18.5±3.6, respectively. The recovery rates of the two groups were 59.9%±36.5% and 59.5%±30.8%, respectively. NRS scores also improved significantly from 5.8±2.4 and 5.8±2.3 to 1.0±1.2 and 0.75±0.83, respectively. However, there was no significant difference between the two groups regarding these operative results (Table 1). During the follow up period, two recurrences were observed in the group that underwent ILA via the shoulder. One patient underwent a second PELD 23 months after the first operation. Another patient recovered by conservative medical treatment.
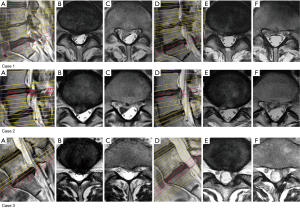
We observed three intraoperative complications only in the group that underwent ILA via the shoulder: persistent numbness in the S1 area, transient muscular weakness, and transient bladder and bowel disturbance (BBD) (Table 2). We retrospectively analyzed these cases. In the former two cases, we preoperatively selected the axilla type; however, after the removal of ligamentum flavum, we first identified the protruded intervertebral disc at the shoulder area and then started the removal from the shoulder area. Although the herniated disc extruded to the caudal and dorsal areas (Figure 2), we might have excessively compressed the nerve root during the manipulation of these extruded fragments. In case of the patient with BBD, LDH was located at mainly the center of the L5/S1 disc level, but her symptom was only left S1 radiculopathy. During the operation, we detected improved MEP in the gastrocnemius muscle (S1), and therefore excessively tried to remove the central part, which did not contribute to the symptoms. Excessive compression of the dural sac might have occurred, with subsequent nerve damage to the corresponding nerves (S2-5).
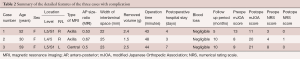
Full table
Discussion
PELD has become a popular operative procedure for the treatment of LDH, especially in the European and East Asian countries. The operative outcome has been differently reported for each operative approach (interlaminar, transforaminal, and posterolateral), and satisfactory results have already been reported (4-9). On the other hand, operative complications and operative techniques to prevent the complications have not yet been fully described (15-19). Furthermore, most of the studies focused on the transforaminal approach, and only a few reports were available regarding the ILA (11,12,20-22). We, therefore, analyzed the results of ILA through two different operative routes: via the shoulder and via the axilla, and investigated the appropriate operative route for ILA to prevent complications.
Preoperative localization of LDH (shoulder, ventral, axilla, and central) is sometimes difficult even with the use of different MRI planes (axial, sagittal, and coronal) or three-dimensional (3D)-MRI (23-25). Especially for large LDH, the diagnosis is more difficult because compressed nerve roots tend to be undetectable. In this study, we preoperatively diagnosed ventral type in 18 cases and operated via the shoulder. Among these cases, large LDH (AP size-ratio >0.5) were noted in 10, in which nerve root retraction was difficult during the operation. Fortunately we did not experience the complication in these cases; we should select ILA via axilla in some of these cases. The direction of the protrusion or extrusion is also an important factor for selection of the operative route. LDH largely extruded in the caudal direction should be treated with ILA via the axilla, as was performed in cases 2 and 3 in this study. Even after exposing the protruded LDH at the shoulder area in the first operative stage, localization of the extruded nucleus at the axilla area should be attempted to find.
It is also difficult to select the operative route for the central type. Excess approach to the central and/or contralateral part may lead to complication not only in the nerve root but also in the dural sac. BBD observed in case 3 in the present study may be due to excessive compression of the dural sac. If space is adequate for removal of LDH at the axilla, ILA via the axilla should be performed. In this study, we did not experience a case presenting both radiculopathy. As MEP of the lower extremity is not useful to detect excessive compression of the dural sac at the L5/S1 disc level, electromyographic monitoring of the anal sphincter muscle may be needed for the central type, which requires decompression of both sides and central part of LDH. Alternatively we should select different operative techniques of PELD being advocated recently (26,27).
In general, the S1 nerve roots more commonly originate above (= cephalic) the L5/S1 disc, thus LDH seems to be located at the axilla more commonly than at the more cephalad levels. Suh et al. reported that 75% of S1 nerve roots originated above the L5/S1 disc in their cadaver study (28). We preoperatively diagnosed 20% of cases of LDH at L5/S1 LDH as the axilla type, and in 18% of these cases, the procedure was performed via the axilla. ILA via the axilla may be performed more commonly in L5/S1 LDH. In at least 3 cases with complications, the procedure should be performed via the axilla. In this study, we divided the surgical results into two groups, with these three cases as the axilla group, and reanalyzed the results. This analysis confirmed that the AP size-ratio of the axilla group was significantly larger than that of the shoulder group (0.51 vs. 0.41: Students’ t-test, P value <0.05). Although other factors were not significant, a large LDH should be considered for ILA via the axilla and the preoperative radiographic findings should be carefully examined.
PELD is a prospective surgical technique in recent years, because of small incision size (8 mm), rapid recovery, short hospital stay, limited blood loss, less destruction of the surrounding tissues, and less postoperative pain. Nevertheless, PELD-specific complications may occur, mainly because of the insertion of operative instruments in a limited operative field. Therefore, the retraction time of the neural structure should be reduced to avoid excess compression. In our study, the pre- and postoperative mean NRS scores were 5.8 and 0.98, respectively. The overall recovery rate of the mJOA score was 59.9%. The follow-up period of our study was short; however, our results are comparable to those of previous studies. To avoid PELD-specific complications, this operative method should be the first line of treatment of LDH.
Conclusions
Preliminary results during a short follow-up period show that ILA of PELD is feasible for the treatment of patients with radiculopathy caused by LDH, especially at the L5/S1 level. However, careful consideration for the selection of the operative route is the most critical to avoid complications.
Acknowledgements
We would like to thank all the operating room staff for their technical assistance, and the medical records clerks who helped collect patient data. We would also like to thank all radiological department staff for accumulating CT and MRI data. This work was partly supported by a grant from the Iwai Medical Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Iwai Medical Foundation and written informed consent was obtained from all patients.
References
- Choi G, Lee SH, Deshpande K, et al. Working channel endoscope in lumbar spine surgery. J Neurosurg Sci 2014;58:77-85. [PubMed]
- Sairyo K, Egawa H, Matsuura T, et al. State of the art: Transforaminal approach for percutaneous endoscopic lumbar discectomy under local anesthesia. J Med Invest 2014;61:217-25. [Crossref] [PubMed]
- Yeung AT. The Evolution and Advancement of Endoscopic Foraminal Surgery: One Surgeon's Experience Incorporating Adjunctive Techologies. SAS J 2007;1:108-17. [Crossref] [PubMed]
- Ahn SS, Kim SH, Kim DW, et al. Comparison of Outcomes of Percutaneous Endoscopic Lumbar Discectomy and Open Lumbar Microdiscectomy for Young Adults: A Retrospective Matched Cohort Study. World Neurosurg 2016;86:250-8. [Crossref] [PubMed]
- Chen HC, Lee CH, Wei L, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar surgery for adjacent segment degeneration and recurrent disc herniation. Neurol Res Int 2015;2015:791943.
- Choi KC, Park CK. Percutaneous Endoscopic Lumbar Discectomy for L5-S1 Disc Herniation: Consideration of the Relation between the Iliac Crest and L5-S1 Disc. Pain Physician 2016;19:E301-8. [PubMed]
- Sinkemani A, Hong X, Gao ZX, et al. Outcomes of Microendoscopic Discectomy and Percutaneous Transforaminal Endoscopic Discectomy for the Treatment of Lumbar Disc Herniation: A Comparative Retrospective Study. Asian Spine J 2015;9:833-40. [Crossref] [PubMed]
- Gadjradj PS, van Tulder MW, Dirven CM, et al. Clinical outcomes after percutaneous transforaminal endoscopic discectomy for lumbar disc herniation: a prospective case series. Neurosurg Focus 2016;40:E3. [Crossref] [PubMed]
- Sencer A, Yorukoglu AG, Akcakaya MO, et al. Fully endoscopic interlaminar and transforaminal lumbar discectomy: short-term clinical results of 163 surgically treated patients. World Neurosurg 2014;82:884-90. [Crossref] [PubMed]
- Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res 1986.37-43. [PubMed]
- Dabo X, Ziqiang C, Yinchuan Z, et al. The Clinical Results of Percutaneous Endoscopic Interlaminar Discectomy (PEID) in the Treatment of Calcified Lumbar Disc Herniation: A Case-Control Study. Pain Physician 2016;19:69-76. [PubMed]
- Li ZZ, Hou SX, Shang WL, et al. The strategy and early clinical outcome of full-endoscopic L5/S1 discectomy through interlaminar approach. Clin Neurol Neurosurg 2015;133:40-5. [Crossref] [PubMed]
- Kim JE, Kim KH. Piriformis syndrome after percutaneous endoscopic lumbar discectomy via the posterolateral approach. Eur Spine J 2011;20:1663-8. [Crossref] [PubMed]
- Baba S, Oshima Y, Iwahori T, et al. Microendoscopic posterior decompression for the treatment of thoracic myelopathy caused by ossification of the ligamentum flavum: a technical report. Eur Spine J 2016;25:1912-9. [Crossref] [PubMed]
- Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices 2012;9:361-6. [Crossref] [PubMed]
- Ahn Y, Lee HY, Lee SH, et al. Dural tears in percutaneous endoscopic lumbar discectomy. Eur Spine J 2011;20:58-64. [Crossref] [PubMed]
- Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery 2015;76:372-80; discussion 380-1; quiz 381. [Crossref] [PubMed]
- Wang H, Zhou Y, Li C, et al. Risk factors for failure of single-level percutaneous endoscopic lumbar discectomy. J Neurosurg Spine 2015;23:320-5. [Crossref] [PubMed]
- Cho JY, Lee SH, Lee HY. Prevention of development of postoperative dysesthesia in transforaminal percutaneous endoscopic lumbar discectomy for intracanalicular lumbar disc herniation: floating retraction technique Minim Invasive Neurosurg 2011;54:214-8. [Crossref] [PubMed]
- Choi KC, Kim JS, Ryu KS, et al. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: transforaminal versus interlaminar approach. Pain Physician 2013;16:547-56. [PubMed]
- Kim HS, Park JY. Comparative assessment of different percutaneous endoscopic interlaminar lumbar discectomy (PEID) techniques. Pain Physician 2013;16:359-67. [PubMed]
- Kim CH, Chung CK, Sohn S, et al. The surgical outcome and the surgical strategy of percutaneous endoscopic discectomy for recurrent disk herniation. J Spinal Disord Tech 2014;27:415-22. [Crossref] [PubMed]
- Byun WM, Ahn SH, Ahn MW. Value of 3D MR lumbosacral radiculography in the diagnosis of symptomatic chemical radiculitis. AJNR Am J Neuroradiol 2012;33:529-34. [Crossref] [PubMed]
- Lee S, Jee WH, Jung JY, et al. MRI of the lumbar spine: comparison of 3D isotropic turbo spin-echo SPACE sequence versus conventional 2D sequences at 3.0 T. Acta Radiol 2015;56:174-81. [Crossref] [PubMed]
- Lou ZH, Qu JR, Li HL, et al. Optimal technique of three-dimensional MRI of the lumbar nerve root and its radicular vein in normal and lumbar disc herniation patients. Chin Med J (Engl) 2011;124:1802-6. [PubMed]
- Choi KC, Kim JS, Park CK. Percutaneous Endoscopic Lumbar Discectomy as an Alternative to Open Lumbar Microdiscectomy for Large Lumbar Disc Herniation. Pain Physician 2016;19:E291-300. [PubMed]
- Lee SH, Choi KC, Baek OK, et al. Percutaneous endoscopic intra-annular subligamentous herniotomy for large central disc herniation: a technical case report. Spine (Phila Pa 1976) 2014;39:E473-9. [Crossref] [PubMed]
- Suh SW, Shingade VU, Lee SH, et al. Origin of lumbar spinal roots and their relationship to intervertebral discs: a cadaver and radiological study. J Bone Joint Surg Br 2005;87:518-22. [Crossref] [PubMed]
Contributions: (I) Conception and design: H Koga; (II) Administrative support: Y Ishii; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: H Koga; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.


