Spinal cord compression from Wegener’s granulomatosis: an unusual presentation
Introduction
Wegener’s granulomatosis (WG) is an incurable form of vasculitis, affecting the small and medium sized arteries and can cause end organ damage hence the patients are on long term immunosuppression (1,2). WG usually affects the upper and lower respiratory tracts and kidneys. The clinical manifestations and symptoms of WG are non-specific and include systemic signs such as fever, malaise, weight loss, arthralgia, and myalgia. Though most patients initially present with upper airway illness, nervous system involvement is frequent in the form of mononeuritis multiplex, peripheral and cranial neuropathy, cerebral infarction or seizures (2,3). Spinal cord involvement is rare (4-6). We present a patient with WG who presented with acute on chronic paraparesis from thoracic spinal cord compression by thickened dura.
Case presentation
A 55-year-old lady known to have WG on prednisolone, and methotrexate regularly and on weekly cycle of intravenous cyclophosphamide, presented with progressive weakness of her right leg of two months duration. She had a mechanical fall a day prior to the presentation, she woke up the following morning unable to mobilise. Her bowel and bladder functions were reasonably preserved. She was brought to the local Accident and Emergency Department.
On examination, the tone in the lower limbs was exaggerated, her power was MRC grade 2/5 from hip downwards; reflexes were brisk and had bilateral ankle clonus. Per rectal examination was normal.
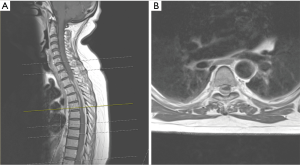
MRI spine (Figure 1) revealed epidural spinal cord compression both from anterior and posterior region at T2–5. This lesion was enhancing on contrast. A similar lesion was at L2 level but not causing neural or thecal sac compression. There was a concern that the lesion at T2–5 could be a hematoma. She underwent emergency surgery where she underwent decompression from T2–T5, there was no extradural haematoma, on the contrary there was a thickened dura (Figure 2), the dura was opened and the thickened portion of the dura excised and synthetic duraplasty was performed.
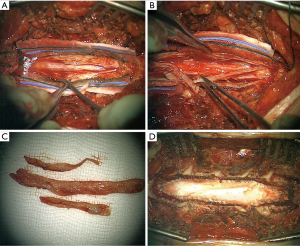
Histopathology showed a thick hyaline collagenous layer consistent with dura, that contains numerous foci of chronic inflammatory cells (including a prominent plasma cell component), areas of sclerosed small vessels at the edge consistent with old granulation tissue, and small foci of necrosis. The pattern of inflammation and necrosis are those WG.
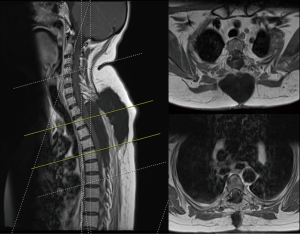
She made a good neurological recovery (Figure 3) and is mobilising independently using a Zimmer frame in a rehabilitation centre.
Discussion
WG, more recently granulomatosis with polyangiitis (GPA) (1), is a systemic disease characterised by necrotising granulomatous inflammation of the upper and lower respiratory tract, glomerulonephritis and vasculitis. Sinusitis is the most common symptom, seen in 73% of patients, and lung disease will develop in 85% of patients during the course of illness. Multifocal necrotizing vasculitis affects the small arteries and veins of the respiratory tract and other sites.
The disease was first described by Dr. Friedreich Wegener in 1936. The association of WG and ANCA (antineutrophil cytoplasmic autoantibody) was first confirmed by Van Der Woude et al. in 1985 (7), currently c-ANCA serves as a sensitive marker for WG. Changes in the levels of ANCA in WG generally reflect disease activity, with increasing titres being a reliable predictor of relapse. The titres of these antibodies decline during treatment, but titres may rise again before a relapse. An immunofluorescent pattern of diffuse cytoplasmic staining has a sensitivity of at least 90% and a specificity approaching 99% for diagnosing generalized WG. An enzyme linked immuno sorbent assay (ELISA) has shown the presence of an antibody that in most cases is directed against a 29-kd serine proteinase (proteinase 3).
Neurologic involvement is not infrequent usually presenting with peripheral neuropathy, particularly mononeuropathy multiplex, sometimes cranial nerve neuropathy is the initial presentation. Drachman reviewed neurologic complications of WG in the literature and found peripheral neuropathy in 21% of cases (3).
He categorized three patterns of neurologic involvement: (I) direct granulomatous involvement; (II) remote granulomatous lesions; and (III) systemic vasculitis. Peripheral neuropathy, including mononeuropathy multiplex, is most likely secondary to systemic vasculitis. Sometimes patients present with sub-acute myelopathy secondary to involvement of the dura.
Brain and meningeal involvement is rare in WG occurring 2% to 8%. Spinal cord involvement is rare. Spinal cord involvement presumably related to either local compression by inflamed tissues or spread of inflammation to adjacent cord and leptomeninges. Kelly et al. (8) illustrated that the underlying pathologic mechanism in WG was extramedullary compression due to extradural and subdural granulomatous involvement of spinal meninges rather than vasculitic meningeal inflammation or cord infarction.
Diffuse cranial dura thickening and contrast enhancement has previously been reported in connection with WG. Results of dural biopsy in previous cases have shown necrotizing granulomata, multinucleated giant cells, and lymphocytic infiltration. Meningeal involvement can be seen as located only in the dura or large focal regions of brain parenchyma adjacent to sites of dural thickening. Dural thickening patterns associated with WG may be symmetric thickening of the entire dura, focal diffuse, focal nodular, and plaque like thickening that has mass effect on brain.
The MRI scan of the signal intensity pattern of the dura lesion was slightly hyperintense on T1-weighted images and prominently hypointense on T2-weighted images, with only minimal enhancement on T1-weighted images after the Intravenous administration of contrast material. These features may arise from paramagnetic components or protein content of the masses (9). The differential diagnosis of dural lesions includes neurosarcoidosis, primary or secondary dural tumours, and infectious meningitis.
In literature there are only a few cases of spinal cord compression. In 2 large series there were only 3 cases of spinal cord compression with one confirmed on histopathology (2,3). Kelly et al. showed that the cord compression was from granulomatous dural involvement than vasculitis (8). Similarly Mantzel et al. showed MRI and histopathological correlation in a case of cervical cord compression (9). Albayram et al. showed a case with cervical and thoracic spinal cord compression (10).
In the case of our patient she was already diagnosed to have WG and was under regular care of the respiratory physicians, the nephrologists and the neurologists. She presented with acute weakness in both her legs and has made a reasonable neurological recovery.
Conclusions
A rare case of WG related thoracic cord compression is presented with literature review.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Falk RJ, Gross WL, Guillevin L, et al. Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis. Ann Rheum Dis 2011;70:704. [Crossref] [PubMed]
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488-98. [Crossref] [PubMed]
- Drachman DA. Neurological Complications of Wegener's Granulomatosis. Arch Neurol 1963;8:145-51. [Crossref]
- Martens J. Spinal cord involvement in Wegener's granulomatosis. Clin Rheumatol 1982;1:221. [Crossref] [PubMed]
- Wang DC, Wei JW, Liu JH, et al. The upper thoracic spinal cord compression as the initial manifestation of Wegener's granulomatosis: a case report. Eur Spine J 2007;16 Suppl 3:296-300. [Crossref] [PubMed]
- Nishino H, Rubino FA, DeRemee RA, et al. Neurological involvement in Wegener's granulomatosis: an analysis of 324 consecutive patients at the Mayo Clinic. Ann Neurol 1993;33:4-9. [Crossref] [PubMed]
- Van Der Woude FJ. Anticytoplasmic antibodies in Wegener's granulomatosis. Lancet 1985;326:425-9. [Crossref] [PubMed]
- Kelly PJ, Toker DE, Boyer P, et al. Granulomatous compressive thoracic myelopathy as the initial manifestation of Wegener's granulomatosis. Neurology 1998;51:1769-70. [Crossref] [PubMed]
- Mentzel HJ, Neumann T, Fitzek C, et al. MR Imaging in Wegener granulomatosis of the spinal cord. AJNR Am J Neuroradiol 2003;24:18-21. [PubMed]
- Albayram S, Kizilkilie O, Adaletli I, et al. MR imaging findings of spinal dural involvement with Wegener granulomatosis. AJNR Am J Neuroradiol 2002;23:1603-6. [PubMed]


