Spontaneous spinal epidural hematoma: literature review
Introduction
Spinal epidural hematomas are a rare occurrence, accounting for less than 1% of all spinal canal space-occupying lesions (1,2). Spontaneous spinal epidural hematomas (SSEH), defined as blood within the epidural space without known traumatic or iatrogenic cause, have an estimated incidence of 0.1 in 100,000 per year (2-5). They may be associated with coagulopathies or arteriovenous malformations (1). SSEHs can produce devastating neurologic deficits (6,7). Appropriate imaging and intervention must be initiated early when a patient presents with symptoms concerning for a SSEH. A paucity of literature regarding this pathologic entity currently exists.
Methods
A literature search was performed using PubMed and Ovid to identify articles pertaining to SSEHs. A review of the literature to date is presented to determine the risk factors, evaluation, and treatment with prognosis.
Results
The literature search revealed a limited number of review articles and scattered case studies regarding SSEHs. The underlying risk factors for SSEHs remain poorly understood. SSEHs present with minimal or no history of antecedent trauma. SSEHs warrant urgent surgical intervention. A case example highlights the rarity of the event and potential pitfalls in diagnosis.
Case example
A 42-year-old male presented to an outside hospital (OSH) due to “pain all over” and “muscle spasms.” His past medical history was significant only for obesity that improved following a vertical sleeve gastrectomy roughly a year prior to presentation, intranasal crystal methamphetamine abuse, and a tooth extraction roughly eight days prior to presentation. He denied any anticoagulant use.
A week prior to presentation, he reported putting a brief case on his left shoulder and a backpack on his right shoulder. When he put the backpack on his right shoulder, he felt a pop in his back that he felt was a “pulled muscle”. He had a stiff and painful neck following this. He began visiting the chiropractor for adjustments due to the pain and was prescribed Naproxen 500 mg 1 tab PO BID.
Four days prior to presentation he noted new and severe, stabbing thoracic and lumbar back pain associated with 2 hours of right lower extremity (RLE) numbness with an unsteady gait when he simply dismounted his motorcycle. He presented the Emergency Department (ED) of the OSH due to this.
Examination by the ED and admitting physicians at that time did not suggest any gross neurological deficits, other than urinary retention. His urinalysis was negative for infection, but a urine drug screen was positive for amphetamines. An MRI of the lumbar spine revealed a small paracentral herniated nucleus pulposus at L5–S1 without gross impingement upon the exiting nerve root. Given that his sensory and motor symptoms to the RLE had resolved, he underwent no further imaging. He was diagnosed with prostatitis as the source of his urinary incontinence—due to concern for infection following the recent tooth extraction. He was discharged several days after presentation with a foley, Flomax 0.4 mg 1 tab PO Qday, and Bactrim 160/800 mg 1 tab PO BID.
Eight hours after discharge, he noted a return of the intense back pain, which was associated with a headache, neck pain, stomachache, and thigh pain. At this time he returned to the OSH ED due to “pain all over” and “muscle spasms”. He, also, now complained of saddle anesthesia. His examination by the consulted neurologist revealed diminished range of motion of his neck with right iliopsoas strength of 4/5. The remaining major muscle groups of the bilateral lower extremities demonstrated 5/5 strength. He complained of hypoesthesia in the right L1–L3 distributions with some diminished perianal sensation but denied any other areas of sensory change. He had a diminished right patella reflex but no upper motor neuron signs. Vitals and laboratory results at that time and while hospitalized at our institution are shown in Table 1.
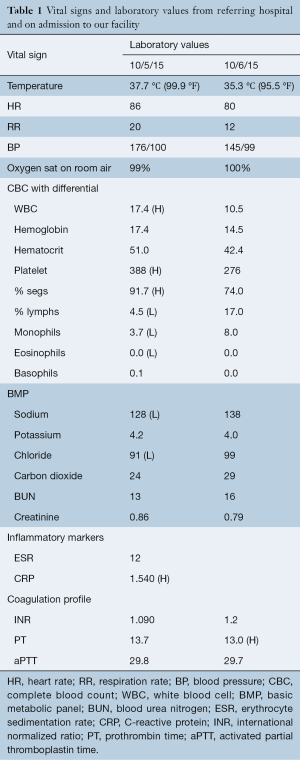
Full table
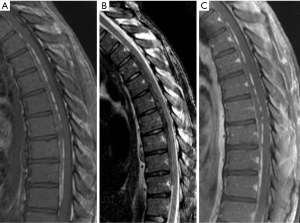
Magnetic resonance imaging (MRI) of the entire neuroaxis, with contrast for the spine images, revealed on T1-weighted imaging a hyperintense lesion in the ventral epidural space from T3–T11 that was hypointense on T2-weighted imaging and did not enhance with gadolinium (Figure 1). There was no evidence of leukomalacia, although there was a slight mass effect particularly at T6–T8. There was concern for an epidural hematoma, and the patient was transferred to our facility for further intervention.
Due to his neurologic changes above and urinary retention, we recommended decompressive surgery. He consented and was taken urgently to the operating room for T6 and T10 hemilaminectomies with irrigation and decompression of the epidural hematoma utilizing pediatric feeding tubes inserted into the canal utilizing the irrigation-suction technique. Intraoperative cultures did not grow any bacteria, and he completed a 24-h, post-operative course of antibiotics.
Six weeks after surgery, his surgical incision had healed, he was neurologically intact, including a full return of bladder function.
Discussion
Patients with SSEHs typically are in their fourth or fifth decades of life (2,3,7), with men slightly more affected than women (1.4:1) (5,7). SSEHs often present with an abrupt onset of severe neck or back pain that can radiate into the extremities and commonly is followed by symptoms ranging from nerve root agitation to full neurologic impairment (1,2-4,7,8). The symptoms typically are that of a lower motor neuron pathology with hyporeflexia and flaccid paralysis (1). There can be a delay in the time from the onset of back pain to neurologic decline, and symptom presentation has been documented to range from within hours to several days or even months from the onset of the back pain (1,3,7). Early suspicion and diagnostic imaging are critical, though, as SSEHs can produce devastating, lasting neurologic deficits ranging from persistent paresis to even death (7).
Trauma and surgery are known risk factors for developing an epidural hematoma (1). The risk factors underlying SSEHs, though, are less well-defined. Many authors suggest an association between SSEHs and arteriovenous malformations, anticoagulant use, underlying coagulopathy, vetebral hemangiomas, and even hypertension (1,2,4,5,7-9). However, a meta-analysis has suggested no increased risk of SSEHs in individuals with hypertension (4). Some have reported upwards of 17–30% of all SSEHs are linked to anticoagulant use (5,8). Others have listed minor trauma, pregnancy, hemophilia, and leukemia as associated causes of SSEH (5,7). However, up to 40–60% of cases demonstrate no identifiable risk factors for the hemorrhage (2,5,7).
The source of bleeding in SSEHs has not been well defined. Authors have postulated that these hemorrhages develop from a rupture of epidural veins, epidural arteries, or a vascular malformation (7). When a patient presents with significant and progressive neurologic decline, the source may be arterial (2). However, most authors support a venous source of bleeding, as the anatomy and sometimes slow progression of symptoms would support a venous source (1-3,6).
The internal epidural plexus that drains the abdomen and thorax is a low pressure, valveless system that may rupture when the pressure is increased from valsalva maneuvers (7). The dorsal epidural space is more often affected by SSEHs than the ventral space (2,4,7). This may be due to the fact that the ventral epidural veins have more support from their position partially under the posterior longitudinal ligament, as well as the fact that the dorsal epidural plexus is larger than the ventral epidural plexus (1,4). In addition, there may also be an area of “locus minoris resistentiae” that is more susceptible to rupture with minor changes in intravenous pressure (1). The higher prevalence of SSEHs in middle-aged individuals may be explained not only by a change in the composition of the vessels but also by the cumulative effect of gravity causing greater dilatation of the vessels (4). Others suggest that the areas of the spine that have more mobility produce more tension on the epidural veins (7), which may explain why SSEHs commonly occur either at the cervicothoracic or thoracolumbar regions (1,2,4,7).
When a SSEH is suspected, the imaging modality of choice is an MRI (7-9). When compared to the spinal cord within 24 h from symptom onset, the hematoma typically appears isointense on T1-weighted and hyperintense on T2-weighted MRI imaging (1,5,7). After 24 h, the hematoma often appears hyperintense on both T1- and T2-weighted images (1,7). Chronic hematomas become hypointense on both T1- and T2-weighted images (1). Fat suppression images may be used to distinguish hematoma from epidural fat (8). Sometimes active bleeding into the hematoma will reveal a central area of enhancement when contrast is used (8). Some authors have noted that occasionally an epidural hematoma will exhibit enhancement, which can be due to hyperemia of the dura or hypertrophic meninges (5,8).
Once diagnosed, urgent surgical intervention is warranted (2,9). Some have suggested that for optimal neurologic improvement, patients should undergo surgical decompression within 12 to 48 h of symptom onset (2,7,8). Yet, some studies have failed to prove a statistically significant difference in outcomes based solely on the time to surgery from symptom onset (9). Given the potential for progressive deficits and that most of the available case reports are based on small numbers, the consensus is for emergent or at least urgent surgical intervention (3,7). Many suggest decompression within 24–36 h of symptom onset for complete deficits and within 48 h for incomplete deficits (3,7). More importantly for long term outcomes, though, is the neurologic status of the patient prior to operative intervention (2,9). This is the most important prognostic indicator (4,5).
Patients who present with more severe symptoms within a shorter time frame tend to have larger hematomas; these are associated with worse outcomes, particularly when four or more spinal segments are involved (4,9). Additionally, a lack of sensory sparing suggests a worse prognosis than an individual presenting with some degree of sensation (4). Areas that have less space available for the cord, i.e., the thoracic spine, do not tolerate hematoma expansion well and portend a worse prognosis (4).
The etiology of the neurologic deficits likely is related not only to compression but also to a secondary inflammatory reaction (4). Thus, even if a patient presents with an ASIA A deficit or in a delayed fashion, surgical intervention is warranted (3,9). Studies have shown that some individuals presenting with an ASIA A deficit have demonstrated recovery even to an ASIA E status following surgical intervention (5). Improvement tends to be more pronounced in the pediatric population (3).
The treatment of choice for SSEHs typically is a hemilaminectomy or a laminectomy followed by irrigation and debridement (3,7,8). If there is an obvious cause of coagulopathy, this should be addressed prior to surgical intervention (5). If a patient is to be treated nonoperatively, the patient needs to be monitored with serial examinations while on strict bed rest (8). Although the hemorrhage may resorb on serial MRI’s even within four months (10,11), the outcomes typically are poor without surgical intervention (10). Thus, non-operative management is reserved only for those who are not surgical candidates or who are asymptomatic (8,9).
For the patient in our case, we provided urgent surgical intervention and noted excellent results. What was interesting in our case presentation, though, was that he developed a SSEH without any documented risk factors but while taking amphetamines. There was no antecedent trauma other than a minor lifting incident, and he denied any anticoagulant use.
The literature would suggest that SSEHs do occur in instances of minimal strain to the spinal cord. Babayev and Eksi documented the case of an 18-year-old female who presented with a thoracic SSEH without any associated trauma, coagulopathy, anticoagulant therapy, or any known risk factors or inciting events for the bleed (3). Moreover, others have noted a SSEH in a high level swimmer, with no known cause other than an aggressive practice the day prior to presentation (12). Certain anatomy may make individuals more susceptible to a SSEH, as was the case in an individual reported by Huang et al. who was noted to have an arteriovenous malformation that precipitated a SSEH after chiropractic treatment (9).
Perhaps with our individual, his first episode of neck pain truly was a neck spasm and when he went to the chiropractor, this was the impetus for his SSEH. However, in the reported instance of SSEH following chiropractic manipulation, the symptoms began immediately following the treatment (6). Our patient did not present immediately after a chiropractic manipulation.
Moreover, the MRI findings in our case were not those usually found in an acute SSEH. This may be due to the fact that our reported patient presented with a subacute on chronic SSEH. Although extremely rare, there have been cases of individuals presenting with recurrent episodes of hemorrhage, with one recurrence roughly a decade later (10) and one repeating at both four and 19 months out from the initial presentation (11).
One other facet of the presented case is the patient’s methamphetamine use. Amphetamine abuse has been associated with coagulopathy (13-17). However, in the reported cases that have led to diffuse intravascular coagulopathy, it typically was associated with renal failure and hyperpyrexia prior to the coagulopathy (14,15,17). Given that our patient did not demonstrate these findings (Table 1), he likely did not ingest enough methamphetamine to produce a coagulopathy. It is possible, though, that he did have an anatomic predisposition—such as an arteriovenous malformation—that may have ruptured in response to the hypertension that can occur with the use of such stimulants (17). Indeed, he did present with hypertension (Table 1). We did not send any specimens from our patient to pathology to assess for an AVM. However, this may suggest that amphetamine use could be a risk factor associated with SSEHs.
Conclusions
The literature describes SSEHs as surgical urgencies that can lead to rapid, permanent neurologic deficits. Although some factors have been described as possible risks for SSEH development, there is no clear consensus. Several case studies report no clear predisposing etiologies in affected individuals. We described the case of a middle-aged male who presented with a massive SSEH after every day activities and concomitant amphetamine use. Perhaps stimulants may increase the potential for a predisposed individual to develop a SSEH. The rarity of this pathology, though, makes it hard to study underlying risk factors. Further study is warranted, but treating physicians should be aware of the subtle signs of SSEH in the setting of minimal or no clear antecedent trauma and should initiate appropriate imaging and treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Al-Mutair A, Bednar DA. Spinal epidural hematoma. J Am Acad Orthop Surg 2010;18:494-502. [Crossref] [PubMed]
- Bhat KJ, Kapoor S, Watali YZ, et al. Spontaneous epidural hematoma of spine associated with clopidogrel: A case study and review of the literature. Asian J Neurosurg 2015;10:54. [Crossref] [PubMed]
- Babayev R, Ekşi MŞ. Spontaneous thoracic epidural hematoma: a case report and literature review. Childs Nerv Syst 2016;32:181-7. [Crossref] [PubMed]
- Bakker NA, Veeger NJ, Vergeer RA, et al. Prognosis after spinal cord and cauda compression in spontaneous spinal epidural hematomas. Neurology 2015;84:1894-903. [Crossref] [PubMed]
- Dziedzic T, Kunert P, Krych P, et al. Management and neurological outcome of spontaneous spinal epidural hematoma. J Clin Neurosci 2015;22:726-9. [Crossref] [PubMed]
- Huang M, Barber SM, Moisi M, et al. Cervical Epidural Hematoma after Chiropractic Spinal Manipulation Therapy in a Patient with an Undiagnosed Cervical Spinal Arteriovenous Malformation. Cureus 2015;7:e307. [PubMed]
- Zhong W, Chen H, You C, et al. Spontaneous spinal epidural hematoma. J Clin Neurosci 2011;18:1490-4. [Crossref] [PubMed]
- Tawk C, El Hajj Moussa M, Zgheib R, et al. Spontaneous epidural hematoma of the spine associated with oral anticoagulants: 3 Case Studies. Int J Surg Case Rep 2015;13:8-11. [Crossref] [PubMed]
- Rajz G, Cohen JE, Harnof S, et al. Spontaneous spinal epidural hematoma: the importance of preoperative neurological status and rapid intervention. J Clin Neurosci 2015;22:123-8. [Crossref] [PubMed]
- Iwatsuki K, Deguchi M, Hirata H, et al. Spontaneously Resolved Recurrent Cervical Epidural Hematoma in a 37-Week Primigravida. Global Spine J 2015;5:e44-7. [Crossref] [PubMed]
- Yamao Y, Takagi Y, Kawauchi T, et al. Surgical management of recurrent spontaneous spinal epidural hematoma with 3 episodes. Spine (Phila Pa 1976) 2015;40:E996-8. [Crossref] [PubMed]
- Fleager K, Lee A, Cheng I, et al. Massive spontaneous epidural hematoma in a high-level swimmer: a case report. J Bone Joint Surg Am 2010;92:2843-6. [Crossref] [PubMed]
- Barrett PJ, Taylor GT. 'Ecstasy' ingestion: a case report of severe complications. J R Soc Med 1993;86:233-4. [PubMed]
- Chadwick IS, Curry PD, Linsley A, et al. Ecstasy, 3-4 methylenedioxymethamphetamine (MDMA), a fatality associated with coagulopathy and hyperthermia. J R Soc Med 1991;84:371. [PubMed]
- Ginsberg MD, Hertzman M, Schmidt-Nowara WW. Amphetamine intoxication with coagulopathy, hyperthermia, and reversible renal failure. A syndrome resembling heatstroke. Ann Intern Med 1970;73:81-5. [Crossref] [PubMed]
- Hahn L. Consumption coagulopathy after amphetamine abuse in pregnancy. Acta Obstet Gynecol Scand 1995;74:652-4. [Crossref] [PubMed]
- Wallace ME, Squires R. Fatal massive amphetamine ingestion associated with hyperpyrexia. J Am Board Fam Pract 2000;13:302-4. [Crossref] [PubMed]
Contributions: (I) Conception and design: All authors; (II) Administrative support: None; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.


