Impact of surgical approach on complication rates after elective spinal fusion (≥3 levels) for adult spine deformity
Introduction
Currently, spinal deformity is estimated to impact 8.8% of the population, equating to approximately 28 million patients (1). As a result, the rate of spinal fusion operations dramatically risen in the past decades, with over a 2.4-fold increase from 1998 to 2008 (1). However, despite the soaring incidence of spinal fusions, controversy persists among spinal surgeons about the risks and benefits associated with different anatomic approaches to fusion. Currently, the operating surgeon dictates choice of approach, and only a few systematic studies have compared the complication profiles associated with different approaches as well as their long-term functional outcomes (2).
Previous studies have demonstrated comparable functional outcomes after anterior and posterior approaches to spinal fusion. Rushton et al. reported similar radiographic results and patient reported outcomes after both anterior and posterior approaches to spinal fusion in a retrospective study of 42 patients with adolescent idiopathic scoliosis (3). Freudenberger et al. reported similar fusion rates between anterior and posterior approaches to interbody lumbar fusion using anterior tension band plating (4). Similarly, in a comparison of anterior and posterior approaches to single-level lumbar fusion, Pradhan et al. reported similar fusion rates and clinical results with both approaches (5). However, little remains known about the implications of different surgical approaches on surgical complications after spinal fusion, especially after fusion involving greater than three levels.
The aim of this study is to determine if there was a difference in intra- and post-operative complication rates between different surgical approaches after elective spinal fusion (≥3 levels) for adult spine deformity.
Methods
In this retrospective study, the medical records of 443 adult (≥18 years old) spinal deformity patients undergoing an elective spinal fusion (≥3) at a major academic medical institution from 2005 to 2015 were reviewed. Institutional review board approval was obtained prior to study initiation. We identified 96 (21.7%) anterior only, 225 (50.8%) posterior only, and 122 (27.5%) combined anterior/posterior approach patients who received a ≥3 spinal fusion (anterior: n=96, posterior: n=225, combined: n=122).
Demographic variables included gender, age, and body mass index (BMI). Co-morbidities included depression, congestive heart failure (CHF), cardiovascular disease (CAD), atrial fibrillation (A-Fib), pulmonary vascular disease (PVD), myocardial infarction (MI), hypertension (HTN), anemia, prior pulmonary embolism (PE), and chronic kidney disease (CKD). Another preoperative variable collected was patient alcohol use. Anatomical location were identified for all cohorts, including cervical only, cervical-thoracic, thoracic only, thoracic-lumbar, thoracic-sacrum, lumbar only, and lumbar-sacrum. Operative variables included the median number of fusion levels (IQR), X-ray imaging, sensory stimulus evoked potentials (SSEP), transcranial motor evoked potentials (TcMEP), electromyography (EMG), fluoroscopy, length of surgery, estimated blood loss, and the number of packed red blood cell (PRBC) transfusions.
Intra-operative complications included the incidence of spinal cord injury, nerve root injury, and durotomy. Post-operative complications included length of hospital stay (LOS), admission to the intensive care unit (ICU), delirium, urinary tract infection (UTI), fever, ileus, deep and superficial surgical site infections (SSI), hypertension, hypotension, hematoma, MI, PE, deep vein thrombosis (DVT), stroke, sepsis, weakness, sensory deficit, urinary retention, discharge with Foley catheter, and rate of 30-Day readmission. The primary outcome investigated in this study was the rate of intra- and post-operative complications.
Parametric data were expressed as means ± standard deviation (SD) and compared via ANOVA. Nonparametric data were expressed as median [interquartile range (IQR)] and compared via the Mann-Whitney U test. Nominal data were compared with the Chi-square test. All tests were two sided and were statistically significant if the P value was less than 0.05. Statistical analysis was performed using JMP®, Version 12. SAS Institute Inc., Cary, NC, 1989–2007.
Results
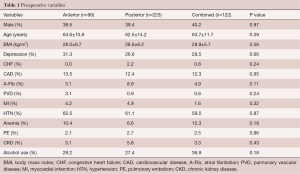
A total of 443 adult patients (anterior: n=96, posterior: n=225, combined: n=122) were included in this study. There were no significant differences in patient demographics between all the cohorts, including male gender (anterior: 38.5% vs. posterior 39.4% vs. combined: 40.2% P=0.97), age (anterior 64.6±10.8 vs. posterior 62.5±14.2 vs. combined: 63.7±11.7, P=0.39), and BMI (anterior: 28.0±6.7 kg/m2vs. posterior: 28.6±6.2 kg/m2vs. combined: 28.9±5.7 kg/m2, P=0.56) (Table 1). There were no significant differences in co-morbidities between all cohorts including depression, CHF, CAD, A-Fib, PVD, MI, HTN, anemia, PE, CKD, and current alcohol use (Table 1).
Full table
Operative complication profile
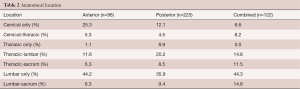
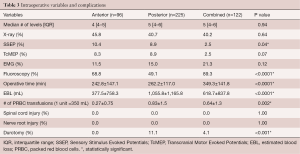
The anatomical locations of the fusions were similar between all groups, with lumbar-only (anterior: 44.2% vs. posterior: 35.9% vs. combined: 44.3%) and thoracic-lumbar (anterior: 11.6% vs. posterior: 20.2% vs. combined: 14.8%) being the most common locations (Table 2). There were no significant differences in the median number of fusion levels [anterior: 4 (IQR 4–5) vs. posterior: 5 (IQR 4–6) vs. combined: 5 (IQR 4–6), P=0.94] and utilization of intraoperative X-Ray, TcMEP, and EMG between the cohorts (Table 3).
Full table
Full table
The posterior approach had significantly higher EBL (anterior: 377.5±758.3 mL vs. posterior: 1,055.8±1,165.8 mL vs. combined: 618.7±837.8 mL, P<0.0001) and number of PRBC blood transfusions (anterior: 0.27±0.75 vs. posterior: 0.83±1.5 vs. combined: 0.64±1.3 P<0.002). The combined approach had a significantly higher operative time (anterior: 242.8±147.1 min vs. posterior: 262.2±117.0 min vs. combined: 349.3±141.8 min, P<0.0001) (Table 3). The posterior approach also had a significantly higher rate of intraoperative durotomies than the other cohorts (anterior: 0% vs. posterior: 11.1% vs. combined: 4.1%, P<0.0001) (Table 3). There were no incidences of spinal cord or nerve root injury within all the cohorts (Table 3).
30-day readmission rates and post-operative complication profile
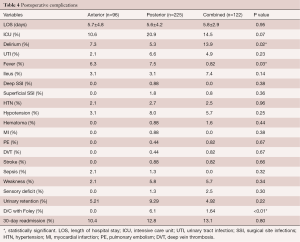
There were no significant differences in the rate 30-day readmissions between the cohorts (anterior: 10.4% vs. posterior: 12.8% vs. combined: 13.1%, P=0.80) (Table 4). Patients in the posterior cohort trended to have increased proportion of patients admitted to the ICU, even though not statistically significant (anterior: 10.6% vs. posterior: 20.9% vs. combined: 14.5%, P=0.07) (Table 4). There were no significant differences in length of hospital stay between the cohorts (anterior: 5.7±4.8 days vs. posterior: 5.6±4.2 days vs. combined: 5.8±2.9 days, P=0.95).
Full table
Postoperative complications were similar between all the groups, with the combined cohort having a significantly higher rate of delirium (anterior: 7.3% vs. posterior: 5.3% vs. combined: 13.9%, P=0.02), while the posterior cohort having a higher rate of postoperative fever (anterior: 6.3%, posterior: 7.5%, combined: 0.82%, P=0.03) and patients being discharged with a Foley catheter (anterior: 0.0 vs. posterior: 6.1% vs. combined: 1.64%, P<0.01) (Table 4).
The prevalence of other post-operative complications was similar between both cohorts (Anterior vs. Posterior. vs. Combined): delirium (7.3% vs. 5.3% vs. 13.9%, P=0.07), UTI (2.1% vs. 6.6% vs. 4.9%, P=0.23), ileus (3.1% vs. 3.1% vs. 7.4%, P=0.14), deep SSI (0.0% vs. 0.88% vs. 0.0%, P=0.38) and superficial SSI’s (0.0% vs. 1.8% vs. 0.8%, P=0.36), HTN (2.1% vs. 2.7% vs. 2.5%, P=0.96), hypotension (3.1% vs. 8.0% vs. 5.7%, P=0.25), hematoma (0.0% vs. 0.88% vs. 1.6%, P=0.44), MI (0.0% vs. 0.88% vs. 0.0%, P=0.38), PE (0.0% vs. 0.44% vs. 0.82%, P=0.67), DVT (0.0% vs. 0.44% vs. 0.82%, P=0.67), stroke (0.0% vs. 0.88% vs. 0.82%, P=0.66), sepsis (2.1% vs. 1.3% vs. 0.0%, P=0.32), weakness (2.1% vs. 5.8% vs. 5.7%, P=0.34), sensory deficit (0.0% vs. 1.3% vs. 2.5%, P=0.30), urinary retention (5.21% vs. 9.29% vs. 4.92%, P=0.22), 30-day readmission (10.4% vs. 12.8% vs. 13.1%, P=0.80) (Table 4).
Discussion
In this retrospective study, we suggest that spinal fusions performed using a posterior-only approach are associated with greater incidence of complications including intraoperative blood transfusions and durotomies compared to anterior-alone or combined anterior/posterior approaches.
Early in the 1960s, Harmon et al. was one of the first to study the relative advantages of anterior compared to posterior approaches to lumbar fusion, citing decreased risk of posterior structure weakening (including durotomy) as well as decreased risk of hemorrhage and LOS to be the principle advantages of anterior approaches (6). However, relatively few subsequent studies have directly compared the results and complication profiles of the approaches. In a retrospective study of 122 patients undergoing single-level lumbar fusion, Pradhan et al. found that posterior approaches were associated with higher incidence of complications including blood loss, number of units transfused, operative time, and LOS (5). In a comparison of 119 ALIF and PLIFs conducted using threaded cylindrical lumbar interbody fusion devices, Scaduto et al. found that posterior approaches are associated with a 4.75 times increased risk of complications (2). Furthermore, the authors found that the posterior approach was associated with significantly higher blood loss, operative time, and incidence of incidental durotomy (2). In a retrospective study of 59 patients with degenerative disk disease undergoing PLIF with pedicle screw instrumentation or ALIF with anterior tension band plating, Freudenberger et al. reported significantly higher blood loss and operative time after PLIF compared to ALIF despite similar functional outcomes with both approaches (4). In a retrospective study of 8,548 patients undergoing 4- to 8-level cervical fusion via either anterior or posterior approaches, Shamji et al. reported that posterior approaches were associated with higher incidence of intraoperative transfusions as well as postoperative infections, hematomas, and respiratory complications (7). These reports parallel the greater EBL and incidence of transfusion and durotomy among the patients undergoing posterior approaches in our cohort.
However, other studies have reported higher complication risk with anterior approaches to spinal fusion. In a retrospective study of 10,941 patients in the MarketScan database undergoing lumbar fusion surgery, Huang et al. found that anterior surgical approaches were associated with increased complications including vascular complications, DVT/PE, and infection (8). In this study, incidence of blood loss/transfusion and durotomy were not examined (8). In a study of 261,356 patients undergoing thoracolumbar spinal fusion from the USA National Inpatient Sample database, Memtsoudis et al. reported that anterior and anterior–posterior fusions were associated with significantly higher complication rates than posterior fusions despite anterior approaches being employed in younger and healthier populations (9). However, in this study, incidence of post-hemorrhagic anemia and need for blood transfusions were significantly reduced in anterior spinal fusions compared to posterior and combined anterior-posterior approaches (9). Similarly, while Goz et al. reported a higher mortality rate for anterior approaches to lumbar interbody fusion compared to posterior approaches, this study also reported greater incidence of neural complications (i.e., durotomy) and acute anemia secondary to hemorrhage with posterior approaches (10). Thus, in spite of discrepancies in overall complication rates among different approaches, greater risk of durotomy and blood loss with posterior approaches is supported across the literature.
The increased risk of incidental durotomy is inherent to the surgical technique employed in posterior approaches, as the posterior approach involves retraction of both the dural sheath and nerve roots. However, the higher blood loss associated with posterior approaches is not easily attributable to technique. Anterior approaches to lumbar fusion typically involve retroperitoneal or transperitoneal approaches performed in close proximity to the ureter, peritoneum, and iliac vessels (2,5). As a result, anterior approaches often involve ligation of sacral and lumbar segmental vessels and require splitting the abdominal muscles and retracting the iliac vessels, posing a higher risk of iliac vessel or presacral plexus damage and resultant hemorrhage compared to posterior approaches (2,5). Patient characteristics and operative variables that pose a higher risk for the different complications associated with each approach should be considered. In a retrospective study of 4,223 patients, Basques et al. identified age >70 years, female sex, pulmonary disease, and preoperative hematocrit <36 as independent risk factors for intraoperative blood transfusion during lumbar fusion using a posterior approach (11). Similarly, in a retrospective study of 1,014 consecutive patients undergoing posterior lumbar fusion, Takahashi et al. identified female sex, older age as well as underlying degenerative spondylolisthesis and juxtafacet cysts as risk factors for incidental durotomy (12). Given the higher risk of blood loss associated with posterior approaches by both our study and the literature (2,4,5), preoperative identification of these risk factors might sway surgical decision-making in favor of an anterior approach. Similarly, demographic variables and underlying spinal pathology influence risk of intraoperative incidental durotomy. Based on these findings, careful preoperative planning about the unique complications associated with different approaches is warranted, especially with regard to the risk of blood loss and incidental durotomy associated with posterior approaches.
An important consideration in preoperative decision-making between different fusion approaches is overall cost. Combined anterior-posterior approaches have been associated with increased operative time in both previous studies and our current report; as a result, combined approach leads to higher operative cost compared to anterior- or posterior-only approaches (10,13,14). Other factors including instrumentation and complication profiles influence the overall costs of the approaches. For example, Shamji et al. reported a 50% increase in cost and 200% increase in hospital stay associated with posterior approaches to cervical fusions compared to anterior approaches (7). In this study, this increased resource utilization was attributed to higher morbidity and incidence of non-routine discharge associated with posterior approaches (7). However, other studies have reported increased resource utilization after anterior compared to posterior approaches (8,10). Further studies are necessary to systematically compare both complication rates and costs associated with fusion approaches. With the current climate of increased emphasis toward reducing excessive healthcare costs, insight into the relative costs of each approach as well as their associated complications could significantly inform preoperative surgical approach decision-making.
This study has limitations, which has implications for its interpretation. First, our small sample size of patients with a documented SSI limits our ability to make any firm conclusions. Our study was performed a single institution and the utilization of the different approaches as well as the surgical technique are subject to the bias of individual surgeons. Although pre- and perioperative variables were prospectively recorded into the study registry at the time of surgery, these variables were retrospectively analyzed for the purposes of this study and as such are subject to the pitfalls associated with all retrospective reviews. Despite these limitations, this study demonstrates that spinal fusions performed using a posterior approach are associated with greater incidence of transfusion and durotomy compared to anter.
Conclusions
In this retrospective study, we suggest that operative approach to spinal fusion may influence surgical outcomes, with posterior approaches leading to increased complication rates compared to the anterior or combined anterior/posterior approaches. In particular, posterior approaches lead to greater incidence of intraoperative durotomies and transfusions. Knowledge of approach-specific complications can inform preoperative approach selection and enhance patient counseling about the unique complications associated with spinal fusion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board approval (approval number: Pro00066331) was obtained prior to study initiation and all patients provided written informed consent.
References
- Rajaee SS, Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67-76. [Crossref] [PubMed]
- Scaduto AA, Gamradt SC, Yu WD, et al. Perioperative complications of threaded cylindrical lumbar interbody fusion devices: anterior versus posterior approach. J Spinal Disord Tech 2003;16:502-7. [Crossref] [PubMed]
- Rushton PR, Grevitt MP, Sell PJ. Anterior or posterior surgery for right thoracic adolescent idiopathic scoliosis (AIS)? A prospective cohorts' comparison using radiologic and functional outcomes. J Spinal Disord Tech 2015;28:80-8. [Crossref] [PubMed]
- Freudenberger C, Lindley EM, Beard DW, et al. Posterior versus anterior lumbar interbody fusion with anterior tension band plating: retrospective analysis. Orthopedics 2009;32:492. [Crossref] [PubMed]
- Pradhan BB, Nassar JA, Delamarter RB, et al. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech 2002;15:355-61. [Crossref] [PubMed]
- Harmon PH. Anterior excision and vertebral body fusion operation for intervertebral disk syndromes of the lower lumbar spine: three-to five-year results in 244 cases. Clin Orthop Relat Res 1963.107-27. [PubMed]
- Shamji MF, Cook C, Pietrobon R, et al. Impact of surgical approach on complications and resource utilization of cervical spine fusion: a nationwide perspective to the surgical treatment of diffuse cervical spondylosis. Spine J 2009;9:31-8. [Crossref] [PubMed]
- Huang KT, Hazzard M, Thomas S, et al. Differences in the outcomes of anterior versus posterior interbody fusion surgery of the lumbar spine: a propensity score-controlled cohort analysis of 10,941 patients. J Clin Neurosci 2015;22:848-53. [Crossref] [PubMed]
- Memtsoudis SG, Vougioukas VI, Ma Y, et al. Perioperative morbidity and mortality after anterior, posterior, and anterior/posterior spine fusion surgery. Spine (Phila Pa 1976) 2011;36:1867-77. [Crossref] [PubMed]
- Goz V, Weinreb JH, Schwab F, et al. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J 2014;14:2019-27. [Crossref] [PubMed]
- Basques BA, Anandasivam NS, Webb ML, et al. Risk Factors for Blood Transfusion With Primary Posterior Lumbar Fusion. Spine (Phila Pa 1976) 2015;40:1792-7. [Crossref] [PubMed]
- Takahashi Y, Sato T, Hyodo H, et al. Incidental durotomy during lumbar spine surgery: risk factors and anatomic locations: clinical article. J Neurosurg Spine 2013;18:165-9. [Crossref] [PubMed]
- Pourfeizi HH, Sales JG, Tabrizi A, et al. Comparison of the Combined Anterior-Posterior Approach versus Posterior-Only Approach in Scoliosis Treatment. Asian Spine J 2014;8:8-12. [Crossref] [PubMed]
- Sponseller PD, Jain A, Newton PO, et al. Posterior Spinal Fusion With Pedicle Screws in Patients With Idiopathic Scoliosis and Open Triradiate Cartilage: Does Deformity Progression Occur? J Pediatr Orthop 2016;36:695-700. [Crossref] [PubMed]
Contributions: (I) Conception and design: AA Elsamadicy, O Adogwa, S Behrens, A Sergesketter, A Chen; (II) Administrative support: AI Mehta, RA Vasquez, J Cheng, CA Bagley, IO Karikari; (III) Provision of study materials or patients: AA Elsamadicy, O Adogwa, S Behrens, A Sergesketter, A Chen; (IV) Collection and assembly of data: AI Mehta, RA Vasquez, J Cheng, CA Bagley, IO Karikar; (V) Data analysis and interpretation: AA Elsamadicy, O Adogwa, S Behrens, A Sergesketter, A Chen; (VI) Manuscript writing: All authors; (VII): Final approval of manuscript: All authors.


