Lateral lumbar interbody fusion with unilateral pedicle screw fixation for the treatment of adjacent segment disease: a preliminary report
Introduction
Adjacent segment degeneration and adjacent segment disease (ASD) were first described by Hilibrand and Robbins in 2004 (1). ASD refers to the development of new clinical symptoms that correspond to the radiographic changes adjacent to the level of a previous spinal fusion. Since this terminology was introduced, our understanding of ASD has evolved, and most authors now accept that this problem is multifactorial in origin (2-5). Harrop et al. reported in a systematic review that lumbar fusion was associated with a 34% and a 14% incidence of adjacent segment degeneration and disease, respectively, at a mean follow up of 7.8 years (6).
Natural history of the adjacent level disc, increased adjacent segment mobility, and disruption of adjacent segment anatomy are thought to contribute towards the development of ASD (2,7-10). Open posterior procedures involving decompression and fusion typically expose and disrupt the normal anatomy of the adjacent level, particularly the ligamentous structures, and are thought to contribute to the development of ASD. Min et al. compared posterior and anterior interbody fusions for spondylolisthesis and found that the incidence of ASD to be 82.6% for posterior lumbar interbody fusion (PLIF) and 44% for anterior lumbar interbody fusion (ALIF) (11). Harrop et al. reported the incidence of adjacent segment degeneration and disease with a posterior procedure to be from 8% to 100%, and 0% to 27.5%, respectively (6).
Lateral lumbar interbody fusion (LLIF) was introduced by Pimenta in 2001 and involves a transpsoas approach to access the anterior vertebral column (12). One of the main potential advantages of this approach in comparison to the traditional vertebral interbody fusion approaches, such as ALIF and PLIF, is the preservation of ligamentous structures and potentially decreased soft tissue exposure and injury (12). In addition to indirectly decompressing the neural structures through interbody distraction, Marchi et al. have recently published that stand alone lateral interbody fusion was associated with a 91% successful fusion rate (13). In a biomechanical cadaveric study, Pimenta et al. have found that a 18 mm extreme lateral interbody cage (XLIF) with unilateral pedicle screw provided greater stability than the 11 mm transforaminal lumbar interbody cage with bilateral pedicle screws (14). Treatment of ASD with LLIF allows for preservation of the anterior and posterior longitudinal ligaments, which preserves stability. The preservation of soft-tissue stabilizing structures may allow less invasive supplemental stabilization techniques, such as unilateral pedicle screw instrumentation. The use of unilateral pedicle screw instrumentation following LLIF for treatment of ASD may reduce the extent of revision surgery while providing adequate stabilization for successful fusion.
The purpose of this study was to report the early clinical and radiographic outcomes of patients who had undergone single-level LLIF using the extreme lateral interbody fusion cage (XLIF, Nuvasive Inc., San Diego, CA, USA) with unilateral pedicle screw instrumentation for treatment of ASD.
Methods
This study was approved by the institutional review board (IRB). Between June 2008 and December 2012, 21 patients underwent 1-level LLIF with unilateral pedicle screw instrumentation for treatment of ASD. One patient was excluded due to incomplete data, leaving 20 total patients included in the analysis. Two patients were fused at L1–2, 5 patients at L2–3, 11 patients at L3–4, and 2 patients at L4–5. Demographics, comorbidities, clinical assessment, perioperative details, and complications were assessed. Oswestry disability index (ODI), short form-12 (SF-12), and visual analog scale (VAS) scores were obtained before surgery and at regular follow-up visits using Phoenix Medcom (Phoenix Medcome Inc. Cortlandt Manor, NY, USA) electronic medical record software and office charts.
Anteroposterior (AP) and lateral (Lat) radiographs were evaluated for fusion, cage migration, and subsidence of the interbody cage into the superior and inferior endplates by one musculoskeletal fellowship-trained radiologist. Successful fusion was defined as bridging trabeculae crossing the adjacent vertebral bodies either through or around the implants and an absence of radiolucent lines around more than 50% of either of the implant surfaces (13). If flexion/extension views were available, additional criteria of less than 5° of angular motion, less than or equal to 3 mm of translation were used. Subsidence was classified as Grade 0 if there was 0–24% loss of post-operative disc height, Grade 1: 25–49%, Grade 2: 50–74%, and Grade 3: 75–100% (13). Radiographs were obtained using Picture Archiving and Communication System (McKesson, San Francisco, California, USA).
Surgical technique
LLIF was performed using a lateral transpsoas approach previously described in institutional studies (12,15). LLIF was performed through a right-sided approach in 7 cases and through a left-sided approach in 13 cases. LLIF cage sizes were 18 mm width for 14 cases (70%) and 22 mm width for 6 cases (30%). Recombinant human Bone morphogenic protein-2 (rhBMP-2) (INFUSE, Medtonic Inc., Minneapolis, MN, USA) with demineralized bone matrix (DBM) putty (Osteotech Inc., Eatontown, NJ, USA) was used to fill the fusion cages in 18 patients (90%). Autograft harvested from the iliac crest with DBM putty was used to fill the fusion cages in 2 patients (10%). Lateral trans-vertebral screws were placed in 2 cases, and an anterior vertebral column plate with trans-vertebral screw fixation was used in 1 case (XLP plate, Nuvasive Inc., San Diego, CA, USA).
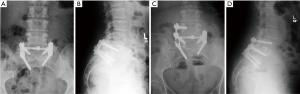
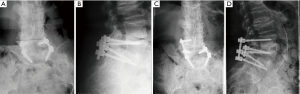
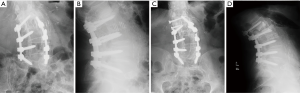
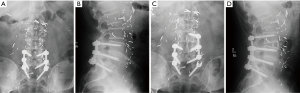
Following LLIF, posterior surgery was performed using a single-sided paramedian Wiltse approach at the level of ASD and the level immediately caudal to the level of ASD. Microsurgical hemilaminectomy decompression was performed in 9 cases (45%). For cases with prior single level pedicle screw instrumentation (35%), the pedicle screw at the cranial-most vertebral level was replaced, an additional pedicle screw was inserted at the level of LLIF, and either a single level rod (n=5) (Figure 1) or double level rod (n=2) (Figure 2) was used. For cases with prior multi-level pedicle screw instrumentation (65%), an additional pedicle screw was inserted at the level of LLIF, and the original rod was either burred at the cranial level with insertion of a new single level rod for the adjacent segment (n=10) (Figure 3) or removed completely with insertion of a new double level rod for the adjacent segment (n=3) (Figure 4). Unilateral pedicle screw instrumentation was placed using direct visualization of bony landmarks and fluoroscopy, 10 on the right side (50%) and 10 on the left side (50%). Posterolateral fusion was performed on a decorticated facet fusion bed using autograft bone with DBM putty in 8 patients (40%) or rhBMP-2 with autograft bone and DBM putty in 12 patients (60%).
Statistical analysis
Continuous variables are presented as mean ± standard deviation. Wilcoxon signed-rank tests were used to compare pre-operative and post-operative clinical outcomes. Statistical analysis was performed using SPSS 20.0.0 (IBM, Armonk, New York, USA). P value less than 0.05 were considered statistically significant.
Results
Patient demographics
The patient cohort included 8 men and 12 women, with an average age of 63.2±13.7 years (range, 41–86 years). Average BMI of patients was 26.5±5.5 kg/m2 (range, 19.5–41.2 kg/m2). Preoperative narcotics were used by 16 patients (80%). The average number of levels previously fused was 1.9±0.8 levels (single level: 8; two level: 7; three level: 5).
Perioperative course
The mean operative time was 214.1±47.2 minutes (range, 146–342 minutes). Average estimated blood loss was 187.5±90.1 cc (range, 50–400 cc). There were no cases of intra-operative complications. No patients received a transfusion during the procedure or postoperative hospital stay. Average length of hospital stay was 4.4±1.7 days (range, 2–9 days).
Clinical indices
Final clinical outcome instruments were taken an average of 13.0±12.7 months post-op (range, 1–49 months). VAS scores improved from pre-op (7.7±1.8 out of 10) to final follow-up (4.3±2.6 out of 10), a significant difference of 3.4 points (P=0.006) (Table 1). ODI scores decreased post-operatively from 48.5±12.0 out of 100 to 40.0±17.4 out of 100, but this improvement was not significant (P=0.181). SF-12 physical health component (PC) score (30.6±8.0 to 31.6±8.9, P=0.480) and SF-12 mental component (MC) score (43.5±13.6 to 45.9±12.5, P=0.937) improved marginally, but these differences were not significant.
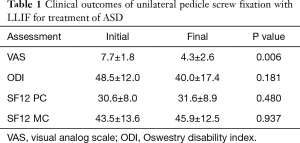
Full table
Radiographic evaluation
Average radiographic follow-up was 15.7±14.2 months (range, 1.5–47.9 months). There were 14 patients (70%) with final follow-up radiographs acquired after minimum 6 months post-op. Out of these 14 patients, there were 13 cases of successful fusion (93%). Subsidence of the adjacent cranial vertebra was Grade 0 in all patients (100%), while subsidence of the adjacent caudal vertebra was Grade 0 in 13 patients (93%) and Grade 1 in 1 patient (7%). There was ventral cage migration ≥3 mm in 3 cases (21%) by measurement of cage marker position relative to adjacent vertebral bodies. There was no evidence of endplate fractures in immediate and final follow-up radiographs.
Complications
There were no cases of intra-operative dural tears or immediate post-operative infections. There were two cases of transient motor weakness (10%). One patient experienced transient weakness in hip flexion (4/5 strength) that resolved by 6 months follow-up. There was one case of transient epidural seroma (5%). Two patients required further surgery for further ASD of the caudal intervertebral segment at 19 and 36 months respectively after the index unilateral pedicle screw and LLIF procedure. For both patients, another interbody cage was placed at the caudal adjacent level and unilateral pedicle screw fixation was placed on the side opposite of the prior unilateral pedicle screw construct (Figure 5). There were no cases of vertebral fracture during follow-up.

Discussion
Surgical treatment of ASD presents many challenges, including high rates of complication due to the need for revision of prior posterior instrumentation (16-18). The principal surgeon hypothesized that the use of LLIF and unilateral pedicle screw fixation may provide sufficient treatment of the symptomatic ASD while minimizing the extent of revision required for surgery and the risk of associated complications. There were no complications during the intra-operative period and hospital stay. Also, during the post-operative course, there was significant improvement in VAS pain scores (P=0.003) with good fusion rates (93%). However, differences in functional outcome scores were not significant (P=0.480 for SF12-PC and P=0.181 for ODI). The non-significant decrease in ODI score from 48.5±12.0 to 40.0±17.4 during final follow-up may suggest a worse natural history of spine pathology in ASD patients. It is possible that patients requiring surgery for ASD may be biased towards a natural history that is predisposed to progressive degenerative spinal diseases with limited potential for recovery from surgical intervention (2). Patients with ASD should be consoled about reasonable expectations from surgery. Previous studies on the treatment of ASD have focused on different surgical approaches, and reported inconsistent clinical and radiographic outcomes, with high rates of complications (16-18). Whitecloud et al. found that decompression and instrumented posterolateral fusion was associated with a 17% pseudarthrosis rate, poor results and complications, which included hardware failure and increased postoperative infection (16). Phillips et al. and Miwa et al. both reported similar outcomes, with good clinical outcomes of 58% and 56% respectively (17,18). In contrast, Cho et al. reported good clinical outcomes in 88.9% of their patients who had undergone a decompression and instrumented posterolateral fusion for ASD (19). Direct comparison of surgical techniques for treatment of ASD should be performed.
ASD is a multifactorial problem, with natural history of the adjacent disc, disruption of the anatomy of the adjacent level with the index surgery, and increased biomechanical stress on the adjacent levels following arthrodesis being the major contributors to the development of ASD (2,7-10). Strategies to avoid ASD in primary surgery include avoidance of injury to the adjacent level soft tissue (2). In this current study, the principal surgeon believed that a less invasive treatment of ASD using LLIF and unilateral pedicle screw fixation would better preserve the ligamentous and soft tissue stabilizing structures, thus minimizing the possibility of further ASD while treating the current symptoms. There were two patients in this current study that developed ASD that required further surgery (10%). Interestingly, Marchi et al. reported a 91% successful fusion rate with standalone LLIF, while Pimenta et al. have demonstrated that the 26 mm width LLIF cage provided greater stability than the 18 mm width LLIF cage (13,14). Standalone LLIF may provide adequate stability for successful arthrodesis while preserving the posterior elements, which may reduce the incidence of ASD. Further study of the impact of minimally invasive techniques on the avoidance of ASD should be explored.
Although LLIF has been effectively used in the setting of many adult degenerative disorders, including degenerative disc disease, degenerative or low grade spondylolisthesis, scoliosis, and now ASD, concerns remain about its safety regarding injury of the lumbosacral plexus as it travels within the psoas muscle. The reported incidence of nerve injury following LLIF ranges from 0.7% to 23%, with the largest series having a minimum follow up of 18 months (20-22). Lykissas et al. recently reported immediate surgery-related sensory and motor deficits of 38% and 23.9% respectively, and anterior thigh/groin pain of 38.5% in a retrospective series of 451 patients (23). These authors identified the L4–L5 level of surgery and the use of rhBMP-2 as risk factors for persistent motor deficits. In this study, 4 patients (20%) developed transient neurological deficits, 2 with surgery at L2–3 and 2 with surgery at L3–4. In our case series, 10% of patients developed anterior groin pain and 10% of patients developed transient motor weakness. The risk of neurological deficits following LLIF should be weighed against the benefits of the procedure.
Cage migration and subsidence are additional concerns of LLIF with unilateral pedicle screw instrumentation. We found that there were 3 cases of ventral cage migration (21%). None of these cases were symptomatic nor did they require revision surgery. Low grade cranial and caudal subsidence was evident in all patients. Pimenta et al. did not find a significant difference in long term clinical outcomes based on grade of subsidence, although they reported a transient acute increased pain in patients with grade 3 subsidence at 6 weeks follow-up (13). Our favorable radiographic and VAS scores suggest that low grade subsidence may not have a negative impact on outcomes. There is a paucity of literature on subsidence in the context of ASD that should be further investigated.
This study has several limitations. Due to the pilot nature of the study, there was a small sample size without any control group. However, early assessment of the technique allows for adjustment and modification. Fusion was assessed by plain radiographs instead of gold standard CT imaging (24,25). Larger prospective studies with longer follow up are required in the future to address treatment and avoidance of ASD.
Conclusions
In conclusion, this study demonstrated that patients with ASD who underwent LLIF and unilateral pedicle screw instrumentation may have significantly reduced pain and favorable radiographic outcomes. However, functional improvement is limited. Further investigation in techniques for treatment of ASD is warranted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by our Institutional Review Board (Approval ID: 2014-097).
References
- Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 2004;4:190S-4S.
- Helgeson MD, Bevevino AJ, Hilibrand AS. Update on the evidence for adjacent segment degeneration and disease. Spine J 2013;13:342-51.
- Sears WR, Sergides IG, Kazemi N, et al. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J 2011;11:11-20.
- Kim KH, Lee SH, Shim CS, et al. Adjacent segment disease after interbody fusion and pedicle screw fixations for isolated L4-L5 spondylolisthesis: a minimum five-year follow-up. Spine (Phila Pa 1976) 2010;35:625-34.
- Kulkarni V, Rajshekhar V, Raghuram L. Accelerated spondylotic changes adjacent to the fused segment following central cervical corpectomy: magnetic resonance imaging study evidence. J Neurosurg 2004;100:2-6.
- Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1701-7.
- Weinhoffer SL, Guyer RD, Herbert M, et al. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine (Phila Pa 1976) 1995;20:526-31.
- Dekutoski MB, Schendel MJ, Ogilvie JW, et al. Comparison of in vivo and in vitro adjacent segment motion after lumbar fusion. Spine (Phila Pa 1976) 1994;19:1745-51.
- Djurasovic MO, Carreon LY, Glassman SD, et al. Sagittal alignment as a risk factor for adjacent level degeneration: a case-control study. Orthopedics 2008;31:546.
- Aiki H, Ohwada O, Kobayashi H, et al. Adjacent segment stenosis after lumbar fusion requiring second operation. J Orthop Sci 2005;10:490-5.
- Min JH, Jang JS. The clinical characteristics and risk factors for the adjacent segment degeneration in instrumented lumbar fusion. J Spinal Disord Tech 2008;21:305-9.
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43.
- Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8.
- Pimenta L, Turner AW, Dooley ZA, et al. Biomechanics of lateral interbody spacers: going wider for going stiffer. ScientificWorldJournal 2012;2012:381814.
- Aichmair A, Lykissas MG, Girardi FP, et al. An institutional six-year trend analysis of the neurological outcome after lateral lumbar interbody fusion: a 6-year trend analysis of a single institution. Spine (Phila Pa 1976) 2013;38:E1483-90.
- Whitecloud TS 3rd, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 1994;19:531-6.
- Miwa T, Sakaura H, Yamashita T, et al. Surgical outcomes of additional posterior lumbar interbody fusion for adjacent segment disease after single-level posterior lumbar interbody fusion. Eur Spine J 2013;22:2864-8.
- Phillips FM, Carlson GD, Bohlman HH, et al. Results of surgery for spinal stenosis adjacent to previous lumbar fusion. J Spinal Disord 2000;13:432-7.
- Cho KS, Kang SG, Yoo DS, et al. Risk factors and surgical treatment for symptomatic adjacent segment degeneration after lumbar spine fusion. J Korean Neurosurg Soc 2009;46:425-30.
- Pumberger M, Hughes AP, Huang RR, et al. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J 2012;21:1192-9.
- Isaacs RE, Hyde J, Goodrich JA, et al. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35:S322-30.
- Lykissas MG, Cho W, Aichmair A, et al. Is There any Relation Between the Amount of Curve Correction and Postoperative Neurologic Deficit or Pain in Patients Undergoing Standalone Lateral Lumbar Interbody Fusion? Spine (Phila Pa 1976) 2013;38:1656-62.
- Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J 2014;14:749-58.
- Cook SD, Patron LP, Christakis PM, et al. Comparison of methods for determining the presence and extent of anterior lumbar interbody fusion. Spine (Phila Pa 1976) 2004;29:1118-23.
- Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol 2005;26:2057-66.


