Navigation accuracy comparing non-covered frame and use of plastic sterile drapes to cover the reference frame in 3D acquisition
Introduction
Improvements in surgical navigation used in conjunction with 3-dimensional (3D) radiograph data have allowed spine surgeons to perform instrumented fusion cases more safely and efficiently. A new draping challenge associated with use of this technological advancement has arisen where surgeons must maintain strict aseptic technique to avoid any potential increase in risk for surgical site infections (SSIs).
Approximately 27 million surgeries are performed each year in the United States with as many as 500,000 resulting in nosocomial SSI (1,2). Because SSIs are responsible for higher mortality and morbidity, it is important for the surgical team to reduce the incidence by implementing strict aseptic principles (3,4). One such aseptic principle is the use of sterile surgical drapes to create an aseptic environment during an operation. The major purpose of draping is to create a barrier of the surgical field from the sources of contamination (5,6). Association of perioperative Registered Nurses (AORN) has established recommendations for draping non-sterile equipment commonly brought in directly over the sterile field.
Because draping is so important, there are specific draping protocols to ensure that the surgical team correctly positions and maintains the draping on the patient during the operation. Furthermore, to enhance efforts to mitigate exogenous sources of contamination, AORN has established evidence-based practice recommendations for draping non-sterile equipment commonly brought in directly over the sterile field, such as microscopy equipment and 2-dimensional or 3-dimensional (2D/3D) radiological devices. As there exists various challenges associated with draping the large 2D/3D radiographic devices, alternative draping methods of protecting the sterile field have been recently developed. Specifically, many surgical teams have opted to temporarily protect the sterile field with a drape rather than drape the 2D/3D device itself.
Makeshift drape methods to protect the patient are fraught with potential contamination. Fitting multiple drapes around the patient in a temporary wrap-like fashion is simply not AORN-compliant as there are breaches in sterility, most apparent during the removal process (7). A lack of standardization exists when utilizing a makeshift drape method which can lead to contamination. Finally, the reference frame attached to the patient’s spine will remain part of the sterile field following removal of a makeshift drape method; therefore, it is critical for sterility of the reference frame to be maintained while 2D/3D acquisition is taking place. The reference frame should not be left extending up through the makeshift drape system, exposed to the unsterile 2D/3D radiographic device above.
As a solution to these issues, the Sterile-Z Patient Drape® (TIDI Products, Neenah, WI, USA) was invented. This 4MM clear film single-drape solution temporarily protects the sterile field while a 2D/3D radiological device (e.g., Medtronic’s O-arm® or Stryker’s/NeuroLogica’s BodyTom®) transiently enters the sterile field for data acquisition. Concomitantly, the Sterile-Z Patient Drape® allows for visualization of the reference frame by the navigation camera while the O-Arm® (Medtronic, Minneapolis, MN, USA) is performing data acquisition. This drape envelopes the patient and incorporates the sensitive wires/tubes under the table (e.g., Foley, neuromonitoring wires) thereby preventing accidental dislodgement while the O-arm enters and is removed from the sterile field.
The primary purpose of this investigation is to evaluate for an increased imaging accuracy error associated with the use of the Sterile-Z Drape® by comparing a ‘no drape’ situation to a ‘drape’ situation while using the Medtronic O-Arm®. The second purpose is to then compare the imaging accuracy error with two different thicknesses of the Sterile-Z Drape®, 2MM and 4MM, while using Medtronic O-Arm® Surgical Imaging and StealthStation® S7® Navigation System (Medtronic, Minneapolis, MN, USA).
Methods
Technical description of Medtronic O-Arm® Surgery Imaging and StealthStation® S7® Navigation System:
The O-arm® contains a digital flat panel detector for optimal image quality. The StealthStation® S7® is equipped with an optical camera designed to localize navigation instruments using infrared light triangulation.
A dynamic reference frame (DRF) is rigidly attached to the patient anatomy where the relative location of the DRF is registered to the patient scan and the space surrounding the DRF becomes a 3D coordinate system.
Experimental protocol
The testing jig was placed on the radiolucent table and the Medtronic passive reference frame was attached to jig (Figure 1). The StealthStation® S7® navigation camera was placed at a clinically significant distance of approximately 4.5 feet from the testing jig. The geometry error of the reference frame was captured and seven 3D acquisitions utilizing the ‘no drape’ configuration were acquired. The O-Arm® gantry location and StealthStation® S7® camera position was maintained and seven 3D acquisitions for each of the alternative 2MM and 4MM drape configurations (Figure 2) were acquired. A new drape was utilized for each of the 2MM and 4MM 3D acquisitions. After all 21 3D scans were complete, the O-Arm® gantry was shifted down the table and point match testing was performed on each scan (Figure 3). Point match testing involved storing all eight test point locations in each scan and subsequently touching the physical locations on the testing jig. The Synergy® software displayed the registration error for each point and these values were documented for all 3D acquisitions.


The O-Arm® gantry was then extended out away from its base approximately 9 inches and the system was realigned around testing jig. Using the Synergy® software tracking view, the StealthStation® S7® camera was repositioned to the ‘Middle’ distance, which is approximately 6.5 feet from the testing jig. Seven additional 3D scan acquisitions were acquired for each ‘drape’ configuration and the point match testing was repeated.
Finally, the O-Arm® gantry was extended out to the maximum distance (18 inches) from its base and realigned the system around the testing jig. Using the Synergy® software tracking view, the StealthStation® S7® camera was repositioned to the ‘Far’ distance, which was approximately 8.5 feet from the testing jig and then seven additional 3D scan acquisitions were acquired for each drape configuration and the point match testing was repeated.
Drape configurations
- ‘No Drape’—3D navigation spin captured without any drape covering the navigation reference frame.
- 2MM ‘Drape’—3D navigation spin captured with 2 mm style drape covering the navigation reference frame. The drape was pulled tight and smoothed out as much as possible to ensure flush contact with the reference frame passive markers.
- 4MM ‘Drape’—3D navigation spin captured with 4 mm style drape covering the navigation reference frame. The drape was pulled tight and smoothed out as much as possible to ensure flush contact with the reference frame passive markers.
Registration error
Registration error was tested on all 3D scan acquisitions for each of the three drape configurations and is calculated using a point matching process. Point match is a two-step procedure in which test points are located on the 3D images in the Synergy® software and the corresponding points are physically touched on the testing jig. The difference between the expected and actual point location determines the registration error. Registration error displayed for each of the eight point locations per 3D scan. After all points were documented, the overall average registration error was calculated for each 3D acquisition. The ‘Max Error’ refers to the point location associated with the highest registration error overall for each 3D scan.
Geometry error
All navigation compatible instrument array dimensions were pre-programmed into the Synergy® application. As soon as a valid instrument array visible to the StealthStation® S7® camera it was automatically monitored and the actual geometry of each array was calculated. Geometry error refers to the difference between expected and actual geometry of each instrument array. All navigable instruments must demonstrate a geometry error of 0.49 mm or less to actively track in the Synergy® software.
The testing jig had a passive reference frame attached and the geometry error was documented before each 3D scan acquisition was performed. Two of the three ‘drape’ configurations cover the reference frame with clear plastic; the geometry error was collected to determine if the drape introduces line of sight distortion in the StealthStation® camera as it detected the instrument array.
Statistical analysis
The primary goal of this study was to quantify the expected measurement error attributable to using the 2MM and 4MM ‘drapes’ compared to ‘no drape’. Two additional experimental factors, camera distance and location of measurement within the relevant spatial region, were investigated for their contribution to measurement error as well. Median and maximum errors were reported as raw summaries of the findings. First, the effect of the location on the testing board was assessed and found to not systematically influence the error magnitudes. Thus, all further analysis treated each data point independently and results are representative of the relevant spatial region of StealthStation® S7® Navigation System. Next, the 2MM and 4MM ‘drapes’ were compared to see if there was a difference in accuracy between the two. If one was found to be optimal, that one would be compared with the measurements using ‘no drape’.
Ultimately, two-factor ANOVA models were used to assess the effect of the drape and camera distance on average measurement error. The interaction term between the two factors was removed from the model if not statistically significant. Post-hoc comparisons among factor levels were made using the Bonferroni method. Statistical significance was declared for P<0.05 and all statistical analyses were performed using IBM SPSS Statistics, Version 20 (Armonk, NY, USA).
Results
Data summary
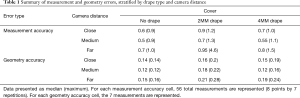
A summary of measurement errors stratified by drape type and camera distance is presented in Table 1. Median (and maximum) measurement accuracy error was higher for the 2MM than for the 4MM drape for each camera distance. The most extreme error observed (4.6 mm) occurred when using the 2MM and the ‘far’ camera distance. However, even when removing this data point, the ‘4MM drape’ consistently outperformed the ‘2MM drape’ with higher measurement accuracy.
Full table
Geometry errors are also presented in Table 1. Although there was some increase in geometry error and its variability induced by both the 2MM and 4MM ‘drape’, the largest observed geometry error (0.28 mm) was well below the 0.49 mm threshold of the measurement system.
Two-factor ANOVA models
Mean measurement error, stratified by drape type and camera distance, is presented in Figure 4. Based on the initial finding that the ‘4MM drape’ exhibited smaller measurement error than the ‘2MM drape’, the ‘4MM drape’ was selected for direct comparison to the ‘no drape’ condition. A 2×3 ANOVA model using the three level camera distance factor was implemented to model measurement error.
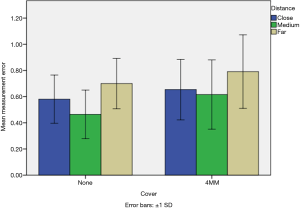
The interaction effect between the ‘drape’ and camera distance was non-significant, {F[2,330]=0.925; P<0.395} and thus a new factorial model was constructed omitting this interaction term. In the final model, the main effects for both drape {F[1,332]=18.2; P<0.001} and camera distance {F[2,55]=23.5; P<0.001} were significant. Specifically, the ‘4MM drape’ induced a mean additional error of 0.11 mm beyond that of the ‘no drape’ condition. Post-hoc comparisons of the camera distance factor showed that the medium distance demonstrated lower average error than both the close distance (mean difference, 0.08 mm; P=0.035) and the far distance (mean difference, 0.21 mm; P=0.001).
Discussion
Reducing the risk of SSI is a primary concern for spine surgeons. Utilization of 3-dimensional radiographic equipment in conjunction with navigation presents a draping challenge. The Sterile-Z Drape® system has been developed to keep the sterile field intact during the usage of such equipment, thereby reducing risk of SSI. This study was designed to determine if light transmission of the navigation camera through the clear film drape would alter accuracy of data acquisition. Specifically, we tested the impact on accuracy of ‘no drape’ condition to the 4MM clear plastic Sterile-Z Drape® ‘drape’ condition, with use of Medtronic O-Arm® Surgical Imaging and the StealthStation® S7® Navigation System. A second purpose of the study was to evaluate whether or not camera distance from the reference frame altered accuracy.
The most significant finding of this investigation is the accuracy error attributed to use of the Sterile-Z Drape® ‘drape’ condition was only 0.11 mm greater than the error associated with the ‘no drape’ condition. Having performed numerous fusion surgeries with Medtronic O-Arm® Surgical Imaging and the StealthStation® S7® Navigation System without any misplaced hardware, the authors of this study believe that such minimal increase in accuracy error is of no clinical significance.
Camera distance from the reference frame is another important variable which has the potential to affect accuracy. In our investigation, data analysis indicates that the medium camera distance induced the least accuracy error of the 3 camera distances tested. In fact, the increased accuracy error of 0.21 mm associated with the far camera distance versus the medium camera distance proved to have almost twice the effect on accuracy as the ‘drape’ condition versus 'no drape' condition (0.11 mm). In addition, the geometry error for all the testing parameters was deemed acceptable by Medtronic standards (<0.5 mm). We recommend using the 4MM drape to protect the surgical field when using computer tomography (CT) and navigational technology.
Acknowledgements
None.
Footnote
Conflicts of Interest: DS Corenman is an occasional teaching consultant for Medtronic with Medtronic Navigation instruments. DS Corenman and EL Strauch own the patent to the Sterile-Z Patient Drape® and have financial ties to TIDI Products, Neenah, WI. E Otterstrom is a member of the Medtronic navigation team (Medtronic did not perform official validation or usability testing for this product). The other authors have no conflicts of interest to declare.
References
- ITC Infection Control Today (2000). Available online: http://www.infectioncontroltoday.com/articles/2000/07/asepsis-and-aseptic-practices-in-the-operating-ro.aspx
- Diaz V, Newman J. Surgical site infection and prevention guidelines: a primer for Certified Registered Nurse Anesthetists. AANA J 2015;83:63-8. [PubMed]
- Labrague LJ, Arteche DL, Yboa BC, et al. Operating Room Nurses Knowledge and Practice of Sterile Technique. J Nurs Care 2012;1:113. [Crossref]
- Osman C. Best Practices Asepsis and Aseptic Practices in the Operating Room; 2000. Available online: http://www.infectioncontroltoday.com/
- AST Association of Surgical Technologies. AST Standards of Practice for Surgical Drapes; 2008. Available online: http://www.ast.org/uploadedFiles/Main_Site/Content/About_Us/Standard_Surgical_Drapes.pdf
- Surgical Specialties. Available online: www.surgicalspecialties.com.au/products/sterile-z-patient-drape#.WamZhT6GO70
- Strauch E, Corenman D, Droy D. Surgical Drape with Separable Elements. United States Patent no. 8,726,907, issued on May 20.


