Morphometric anatomy of the lumbar sympathetic trunk with respect to the anterolateral approach to lumbar interbody fusion: a cadaver study
Introduction
Minimally invasive lateral lumbar interbody fusion (LLIF) techniques have been associated with fewer complications than anterior and posterior lumbar approaches (1). However, the standard lateral approach, which traverses the psoas muscle (i.e., transpsoas approach), has its own limitations and complications (2-6). Of most concern is the potential for injury to the lumbar plexus, minimized by using intraoperative neuromonitoring (7). An alternative approach, anterior to psoas (ATP), without neuromonitoring, has been described (8-11). This approach is also promoted by Medtronic in a technique called “OLIF25” (10). Theoretically, ATP approaches have the benefits of reduced complication rates to the lumbar plexus, compared with the transpsoas approach. In addition, it has the benefit of allowing technically easier access to the L4/L5 disc space, which may be obstructed by the iliac crest in the transpsoas approach (12). Yuan et al. described a similar muscle-sparing approach, which had a reduced complication rate compared with a standard transpsoas approach (13). These techniques traverse a corridor ATP in which the principle longitudinal anatomical structure is the LST. Segmental vessels traverse this region in a medial to lateral direction. Other structures in this space include lymphatics and small sympathetic fibres, for example grey and white rami communicantes and post-ganglionic branches to the midline plexus.
As the LST represents a structure at risk, and a potential impedance to this approach, a more detailed anatomical appreciation of the position of the sympathetic trunk, especially in the lower lumbar spine (L4/5 and L5/S1) is required. Most of the approaches in which the LST is described in the literature are related to research papers in anesthesiology in the context of percutaneous ablative procedures, in which the position of the sympathetic trunk was examined in relation to psoas, rather than in relation to the disc space.
From experience with over 100 ATP surgical cases (Seex and Gragnaniello, unpublished results), we have noted that the LST is always identifiable in the surgical field, and may require retraction anteriorly during the operation (11). Unilateral iatrogenic injury to the lumbar sympathetic trunk (LST) produces a ‘sympathectomy effect’ with vasodilation-induced warmth and oedema in the affected limb. Other documented iatrogenic complications have not been observed in our patients, namely post-sympathectomy neuralgia and compensatory hyperhidrosis (14-16). A bilateral high lumbar sympathectomy may lead to retrograde ejaculation because the sympathetic outflow to the internal vesical sphincter is from L1–L2 (17,18). Topographically, the LST is described as resting on the anterolateral surface of the lumbar vertebrae, following the lateral border of the anterior longitudinal ligament (ALL) and the medial border of the psoas major. Osteophytes may displace the LST, either laterally, or medially (19). The LST lies anterior to the lumbar segmental vessels and has a variable relationship with the tributaries of the iliolumbar veins in the parasagittal (anteroposterior) plane. On average, there are four ganglia present in the LST. Some authors have described specific locations for those ganglia. Others who have reported topographical variability of the ganglia considered these specific descriptions impractical (20). The ganglia may be fused with the lumbar vertebral bodies. Feigl and colleagues observed that the LST’s ‘fixation’ was due to a ‘dense fascia-like connective tissue’. They postulated that it was associated with a continuation of the psoas fascia (19). In contrast, Edwards described the LST as being ‘clothed’ by the ALL (20).
Embryologically, the sympathetic trunk is derived from truncal neural crest cells which migrate ventrally through the anterior portion of the sclerotome, to reside in the para-aortic region dorsolateral to the aorta. Edwards postulated that the lumbar arteries prevent the sympathetic chain from migrating medially. This may be correct, not because the arteries create a physical barrier which prevents the sympathetic fibres migrating too medially, but because, during embryonic development, blood vessels produce neurotrophin NT-3, which induces developing sympathetic fibres to grow upon their surfaces as they distribute from, and to, their smooth muscle targets. In the experimental setting, it has been shown that the LST, and other sympathetic fibres, grow along vasculature to reach their various splanchnic targets, and in the experimental setting, absence of neurotrophins prevented sympathetic fibres reaching those targets (21). Furthermore, sympathetic fibres have been shown to express trophic factors which cause them to grow and branch during ‘target innervation’ (22).
Previous morphometric studies of the LST have used different landmarks to locate the LST according to the procedure being performed. Gu et al. characterised LST morphometry for the purpose of posterolateral lumbar disc approaches using the transverse process as the reference landmark (23). Similarly, Feigl et al. used the medial border of psoas for use in lumbar sympathetic blocks (24). They reported that osteophytes caused the LST to be displaced medially, laterally, or anteriorly—stretched over the surface of the osteophyte.
Our study was performed to characterize the morphometry of the LST for surgical application in an ATP lumbar intervertebral (IV) disc approach.
Methods
Twenty-four embalmed cadavers were selected. Two were excluded due to significant pathology involving the relevant anatomy (n=22, 13 males and 11 females, age range: 50–89, median age: 78). Cadavers were obtained through the Bequest Program of the Anatomy Department at the University of New England. Cadavers were embalmed using a ‘formalin-mix’ solution and prepared by evisceration of abdominopelvic contents; horizontal section through thoracic vertebra 12 (T12) body; and bilateral oblique section of the lower limbs in the inguinal region—thus isolating the posterior abdominopelvic wall. The retroperitoneal ‘great vessel space’ was dissected to expose the LST. All intervening structures were removed. The position of the LSTs were confirmed through the identification of their grey rami communicantes. The midsagittal plane of the lumbar vertebrae was estimated visually, and 14-gauge needles were placed into the IV discs at L3/4, L4/5 and L5/S1 as reference markers. Callipers were used for direct visual measurement of the distance of the LST from the reference marker in the midsagittal plane. Size of the ganglion, adherence of the LST to the disc space, and any osteophytic pathology were recorded. All measurements were performed by authors C Gragnaniello and G Rutter, and verified by K Seex and F Stewart.
Fourteen cadavers were then selected for data validation by CT imaging. The fourteen-gauge reference marker needles were left in situ at the midsagittal point of the IV disc, and others were placed bilaterally along LST at each level. Those needles were used as radio-opaque markers (Figure 1). Linear distances were measured using OSIRIX by author G Rutter and confirmed by author C Gragnaniello.
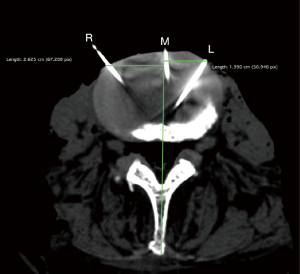
Data was analyzed using Microsoft Excel. Data validation was determined by absolute error and precision at each vertebral level for each side. Error was calculated as the absolute value of the difference between the calliper and CT measurements. Precision was calculated using a two-sample F test for the equivalence of variances (25). The measurements were considered ‘precise’ if the F test was statistically insignificant (i.e., the null hypothesis that the variances are equivalent is true).
Results
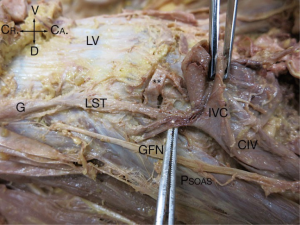
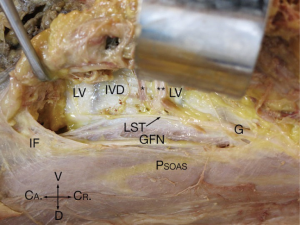
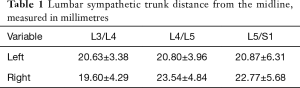
In the 24 specimens, we analyzed 48 LSTs approaches from either the right (Figure 2) or the left (Figure 3). The LST descended vertically from L4 to S1 in 6 specimens (4 left; 2 right). In 14 specimens, the LST had a lateral to medial direction (8 left; 6 right). In the majority of specimens, the LST ran from medial to lateral (N=28; 13 left; 15 right). The mean distance of the LST from the midline at the L3/L4 level was 20.63±3.38 mm (left), and 19.60±4.29 mm (right); at L4/L5 level, 20.80±3.96 mm (left), and 23.54±4.84 mm (right); and at L5/S1 level, 20.87±6.31 mm (left), and 22.77±5.68 mm (right) (Table 1).
Full table
In six specimens, osteophytes were observed at L5/S1. In three specimens (3/6) the LST was stretched upon the surface of the osteophyte. In three specimens, the LST was substantially displaced to one side. In two specimens, the LST was displaced twelve, and fourteen mm lateral to the proximal position it occupied at L4/5. In one specimen, the LST at L5/S1 was 12 mm medial to its proximal position at L4/5—a deviation from the midline of 25 mm lateral to midline, to 8 mm lateral to the midline.
Presence of osteophytes was observed at the L4/5 level in eight specimens. In seven of those, specimens, the LST deviated laterally. In one specimen the LST was stretched over the osteophyte. Where LST was lateral to the osteophyte, the distance between it and the osteophyte was 4–6 mm lateral, when compared to the LST’s position at the level above in six specimens. In one specimen, the LST’s deviation with respect to the osteophyte was 11 mm.
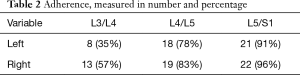
From intraoperative experience, the LST is at times loose over the vertebral body and disc, while in other patients, it is tightly adherent to the disc space, bound by a dense layer of connective tissue. In the 48 specimen sides which we inspected, we confirmed that connective tissue ‘fixation’ of the LST occurred (Table 2) and we observed that even when the LST was ‘free’ over the disc spaces of L3/4 (45%), it tended to be adherent to the L4/L5 (80%), and L5/S1 (93%) disc spaces.
Full table
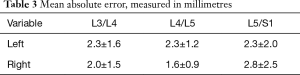
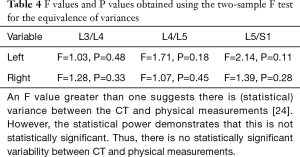
The mean absolute error was calculated as the absolute value of the difference between the physical and corresponding CT measurements. These ranged from 1.6±0.9 to 2.8±2.5 mm, depending on the level (Table 3). None of the F values (ranging from 1.03 to 2.14) were statistically significant (P values between 0.11 and 0.48) (Table 4).
Full table
Full table
Discussion
Anterolateral surgical approaches which avoid psoas muscle-splitting, utilize the natural window created by the anterior border of psoas, and the lateral extent of the great vessels which provide the surgical access corridor. This ‘surgical corridor’, oblique to the midsagittal plane of the lumbar spine, permits the surgeon to place a prosthetic cage into the disc space. The technique has been described by Seex (2,11), Aghayev (9), and Kanno (10).
The proposed advantages of the oblique approach allow for discectomy and cage insertion through the oblique corridor with lesser manipulation required of the major vessels and important anatomical structures (26,27). This corridor is bound by the psoas muscle laterally and aorta and inferior cava or common iliac vessels medially. Cadaveric investigations by Davis et al. (28) and Molinares et al. (29) demonstrate an adequately sized, left-sided oblique corridor at L2-L5, which could be widened via lateral decubitus positioning and the use of approach retractor systems. The prior study (28) reported left oblique corridor sizes for L2/L3, L3/L4, L4/L5 whilst the latter (29) reported measurements of these levels in addition to L1/L2 and L2/L3. Both studies were consistent in demonstrating that the larger corridors were available in the upper lumbar levels, and has been attributed to the conical morphology of the psoas muscle. No significant association was found between corridor sizes with gender. Indeed, these morphometric studies are in keeping with the clinical experience by initial ATP/OLIF studies (2,6,11), where a left sided approach is preferable to all but L5/S1 disc space, as a right sided approach is more likely to require mobilization of the inferior vena cava. At L5/S1 a right sided approach may be preferred as the right common iliac vein is typically more lateral and unlike the left, rarely adherent to the spine or disc space. The position of the LST places it at risk in either left or right sided approaches.
The influence of age should not be underestimated when considering the position of the lumbar plexus during mobilization of the psoas muscles. It has been suggested that in elderly individuals, the atrophied psoas muscle may alter the position of the lumbar plexus (30), and as such increased care should be taken when retracting psoas in the elderly population. Certainly, mobilization of the LST can be difficult in the more degenerative spine because of its adherence to the disc space, vertebrae and osteophytes as seen in this study.
While preservation of the LST is desirable, the consequences of its inadvertent or deliberate sacrifice is rarely a problem at least in the elderly. Reports of the natural history of LST injury in surgical fusion are minimal, and more clinical information is needed to clarify in whom and how significant this is likely to be. The consequences of LST injury or sacrifice in the elderly population appear at least anecdotally to be less critical, possibly given the likely reduced effectiveness of the autonomic system with age and increased rates of diabetic or other neuropathies. By contrast, preservation of the LST in a younger population is important, but can be easier given that younger patients are less likely to have osteophytes. Comparison with alternate approaches to ATP (e.g., transpsoas or ALIF), which in theory have less risk of LST injury, but perhaps more other complications, is desirable. In the authors views, the advantages to the patient of multiple interbody correction from an anterolateral approach outweigh the risk and consequences of LST injury.
Although not the focus of the present morphometric study, there are other structures of importance close to the spine when considering the surgery through the oblique corridor. There have been reported cases where the kidney, renal vasculature or liver partially obstruct the oblique corridor. In a morphometric analysis by Liu et al. (31), authors assessed imaging data from 60 adults to determine features of the vascular window at each lumbar level and proportion obstruction by renal artery and vein positioning. The authors noted that the renal artery and vein were overlapping in 51.7% of cases, including 18.3% in front of the L1/2 IV space. The position of the ureter relative to the oblique corridor is variable but typically is adherent to the peritoneum and is like the other structures mentioned above, retracted with the peritoneum, and not of great concern during the oblique approach being retroperitoneal. The genitofemoral nerve arising from L1 and L2 roots, emerging from psoas around L3 body, is the sensory nerve most at risk, being on the psoas and immediately under the retractor blade during surgery. The venous anatomy, particularly the iliolumbar veins and ascending lumbar veins, should also be mentioned as potentially at risk in the anterolateral and transpsoas procedures. The majority of patients have a single iliolumbar vein, although multiple can be found in up to 25.8% of cases (32), which the operator should be aware of. Typically seen mid body of L5, occasionally the authors have noted the iliolumbar vein to cross the disc space. One cadaveric study (33) found that 5% of LLSVs ran superficial to the obturator nerve whereas 66.6% ran superficial to the LST. The common iliac and both iliac arteries frequently become tortuous with age, and thus have variable position, passing anterior in 70% of cases and posterior to the obturator nerve in 30% of cases in one cadaveric study (34).
Conclusions
With the development of lumbar fusion techniques which utilize an oblique corridor and the retraction of psoas muscle, LST has become an important neural structure to define, protect and mobilize. This paper adds more specimens (n=24) to the literature. The position has been identified and quantified, and this paper notes variations, particularly distortions caused by degenerative processes. In this study, the LST ran in a medial to lateral direction from L3 to S1, and osteophytes typically displace and adhere to the LST.
Acknowledgements
The authors acknowledge the generosity of the University of New England Department of Anatomy body donors and their families, the assistance of Ms. Vicki Weaver and medical student laboratory staff, and Professor Geetha Ranmuthugala for providing statistical expertise. Funding was provided by the Anatomy Department of the University of New England.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics waived as this was a cadaveric lab study with no patients involved.
References
- Patel VC, Park DK, Herkowitz HN. Lateral Transpsoas Fusion: Indications and Outcomes. ScientificWorldJournal 2012;2012:1-6. [Crossref] [PubMed]
- Gragnaniello C, Seex K. Anterior to psoas (ATP) fusion of the lumbar spine: evolution of a technique facilitated by changes in equipment. J Spine Surg 2016;2:256-65. [Crossref] [PubMed]
- Hijji FY, Narain AS, Bohl DD, et al. Lateral lumbar interbody fusion: a systematic review of complication rates. Spine J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J 2017;17:545-53. [Crossref] [PubMed]
- Phan K, Rao PJ, Scherman DB, et al. Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J Clin Neurosci 2015;22:1714-21. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Bendersky M, Solá C, Muntadas J, et al. Monitoring lumbar plexus integrity in extreme lateral transpsoas approaches to the lumbar spine: a new protocol with anatomical bases. Eur Spine J 2015;24:1051-7. [Crossref] [PubMed]
- Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J 2012;6:89. [Crossref] [PubMed]
- Aghayev K, Vrionis FD. Mini-open lateral retroperitoneal lumbar spine approach using psoas muscle retraction technique. Technical report and initial results on six patients. Eur Spine J 2013;22:2113-9. [Crossref] [PubMed]
- Kanno K, Ohtori S, Orita S, et al. Miniopen Oblique Lateral L5-S1 Interbody Fusion: A Report of 2 Cases. Case Rep Orthop 2014;2014:1-5. [Crossref] [PubMed]
- Gragnaniello C, Seex KA. Anterior to psoas fusion of the lumbar spine. Neurosurg Focus 2013;35:Video 13.
- Moro T, Kikuchi SI, Konno SI, et al. An Anatomic Study of the Lumbar Plexus with Respect to Retroperitoneal Endoscopic Surgery. Spine 2003;28:423-8. [Crossref] [PubMed]
- Yuan PS, Rowshan K, Verma RB, et al. Minimally invasive lateral lumbar interbody fusion with direct psoas visualization. J Orthop Surg Res 2014;9:20. [Crossref] [PubMed]
- Buche M, Randour P, Mayne A, et al. Neuralgia Following Lumbar Sympathectomy. Ann Vasc Surg 1988;2:279-81. [Crossref] [PubMed]
- Rieger R, Pedevilla S, Pöchlauer S. Endoscopic Lumbar Sympathectomy for Plantar Hyperhidrosis. J Vasc Surg 2010;52:252. [Crossref]
- Litwin MS. Postsympathectomy Neuralgia. Arch Surg 1962;84:591. [Crossref]
- Sasso RC, Kenneth Burkus J, et al. Retrograde Ejaculation After Anterior Lumbar Interbody Fusion. Spine 2003;28:1023-6. [Crossref] [PubMed]
- Rieger R. Video-assisted retroperitoneoscopic lumbar sympathectomy*. Eur Surg 2012;44:10-3. [Crossref]
- Feigl GC, Kastner M, Ulz H, et al. Topography of the lumbar sympathetic trunk in normal lumbar spines and spines with spondylophytes. Br J Anaesth 2011;106:260-5. [Crossref] [PubMed]
- Edwards EA. Operative Anatomy of the Lumbar Sympathetic Chain. Angiology 1951;2:184-98. [Crossref] [PubMed]
- Kuruvilla R, Zweifel LS, Glebova NO, et al. A Neurotrophin Signaling Cascade Coordinates Sympathetic Neuron Development through Differential Control of TrkA Trafficking and Retrograde Signaling. Cell 2004;118:243-55. [Crossref] [PubMed]
- Ryu YK, Collins SE, Ho HY, et al. An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Dev Biol 2013;377:79-89. [Crossref] [PubMed]
- Gu Y, Xu R, Ebraheim NA, et al. The quantitative study of the lateral region to the lumbar pedicle. Surg Neurol 1999;52:353-6. [Crossref] [PubMed]
- Feigl GC, Kastner M, Ulz H, et al. The lumbar sympathetic trunk: its visibility and distance to two anatomical landmarks. Surg Radiol Anat 2013;35:99-106. [Crossref] [PubMed]
- Langenberg P, Klugh H. Statistics: The Essentials for Research. Technometrics 1988;30:459. [Crossref]
- Li JX, Phan K, Mobbs R. Oblique Lumbar Interbody Fusion: Technical Aspects, Operative Outcomes, and Complications. World Neurosurg 2017;98:113-23. [Crossref] [PubMed]
- Phan K, Maharaj M, Assem Y, et al. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF). J Clin Neurosci 2016;31:23-9. [Crossref] [PubMed]
- Davis TT, Hynes RA, Fung DA, et al. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position: an anatomic study. J Neurosurg Spine 2014;21:785-93. [Crossref] [PubMed]
- Molinares DM, Davis TT, Fung DA. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs: an MRI study. J Neurosurg Spine 2015. [Epub ahead of print]. [PubMed]
- Davis TT, Bae HW, Mok JM, et al. Lumbar plexus anatomy within the psoas muscle: implications for the transpsoas lateral approach to the L4-L5 disc. J Bone Joint Surg Am 2011;93:1482-7. [Crossref] [PubMed]
- Liu L, Liang Y, Zhang H, et al. Imaging Anatomical Research on the Operative Windows of Oblique Lumbar Interbody Fusion. PLoS One 2016;11:e0163452. [Crossref] [PubMed]
- Nalbandian MM, Hoashi JS, Errico TJ. Variations in the Iliolumbar Vein During the Anterior Approach for Spinal Procedures. Spine (Phila Pa 1976) 2013;38:E445-50. [Crossref] [PubMed]
- Unruh KP, Camp CL, Zietlow SP, et al. Anatomical variations of the iliolumbar vein with application to the anterior retroperitoneal approach to the lumbar spine: a cadaver study. Clin Anat 2008;21:666-73. [Crossref] [PubMed]
- Teli CG, Kate NN, Kothandaraman U. Morphometry of the iliolumbar artery and the iliolumbar veins and their correlations with the lumbosacral trunk and the obturator nerve. J Clin Diagn Res 2013;7:422-6. [PubMed]


