Unusual cauda equina syndrome due to multifocal ependymoma infiltrated by lymphoma
Introduction
Ependymoma is a rare tumour of the central nervous system (CNS) located commonly in the brain, the spine or the cauda equina. Ependymoma of the filum terminale is the less common subtype compared to ependymomas encountered in the brain and intramedullary. A grading from the latest World Health Organization (WHO) classification of tumours classifies ependymomas into grade 1 as subependymomas and myxopapillary ependymomas most frequent subtype in cauda equina, conus medullaris or filum terminale. The WHO grade 2 are ependymomas, WHO grade 3 are anaplastic ependymomas. Recently, a new subtype has been added, genetically defined as ependymoma, RELA fusion-positive as grade 2 or 3 (1). The clinical presentation is a progressive neurological deficit with bladder dysfunction and radiculopathy. Myxopapillary ependymomas could be cured by complete removal of the tumour including its capsule when possible. For WHO grade II, adjuvant radiotherapy could be required. Chemotherapy is reserved for uncommon disseminated ependymomas in cerebrospinal fluid (CSF) (2). Primary CNS lymphoma is an extremely rare tumour and affects generally the brain. Non-Hodgkin lymphoma of the CNS is found in no more than 1.3% of the cauda equina tumours and has rarely been reported in literature. The case of concomitant ependymoma infiltrated by primary CNS lymphoma has never been reported.
Case presentation
A 62-year-old man presented to our institution complaining with progressive weakness of his right lower extremity, with numbness and paresthesia of both feet. He had been treated 12 years ago with cystoprostatectomy and ureterostomy for bladder carcinoma, with post-operative urinary incontinence. The patient was suffering from 1 month of right sciatica. Clinical examination showed bilateral numbness of the feet and right-sided paresis severely disabling his gait; rectal tone was preserved. Lumbosacral MRI weighted in T1, T2; T1 without and with gadolinium, and FAT SAT sequences was obtained in emergency. It displayed two intradural extramedullary lesions, well defined at lumbar level, compressing the cauda equina. The first one was located posteriorly to vertebral body of L1–L2, with hyper intense T2 signal, isointense on T1-weighted images highly enhanced after gadolinium. Second one was spreading from L4 to S2 presenting a mucoid cyst in its superior part and an inferior solid part, with hyper intense T2 signal, isointense on T1-weighted with high and homogeneous contrast enhancement. Imaging found three additional intradural, extramedullary tumour formations, with hyper intense T2 signal with contrast enhancement, located in C5–C6, C7 and T4 without mass effect on the spinal cord (Figure 1). Brain MRI didn’t find any primary lesion at cerebral level indicating metastasis.
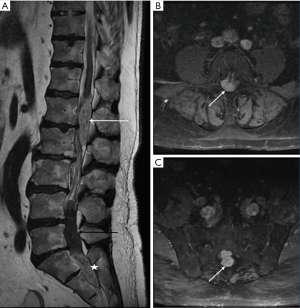
We performed a L1–L2 laminectomy in emergency. After dural opening under microscope, an underlying tumour firmly attached to spinal nerves was encountered. Total removal was obtained by gentle dissection. Because of the presence of another tumour, the procedure was immediately completed with a L5–S1–S2 laminectomy. Intradural examination found the same grayish tumour comprising nerve roots, which was resected in the same way, avoiding neural tissue and allowing subtotal removal.
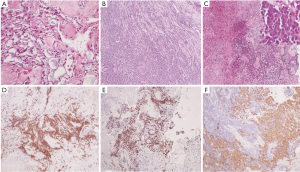
Histological examination found tumour characteristics of myxopapillary ependymoma for the L1–L2 lesion with proliferation of spindle-shaped cells or stellate, with thickened vascular wall by fibrohyalinosis and myxoid material. The tumour cells were immunoreactive for glial fibrillary acidic protein (GFAP) which is an intermediate filament protein that is expressed by astrocytes and ependymal cells, and S-100 protein (present in cells derived from the neural crest) but they did not express cytokeratin and epithelial membrane antigen (EMA) which can differentiate metastatic carcinoma deposits. This tumour did not show proliferative characteristic according to the MIB1 (Ki67) index (marker of cell proliferation correlated to clinical course of cancer). On the other hand, S1-S2 tissue samples displayed the same characteristics of myxopapillary ependymoma with lymphoid proliferation infiltrating the tumour and nerve sheath. Immunochemistry analysis exhibited positivity for CD45, CD20, CD79A staining and BCL2 which are specific of lymphocyte cells and B-cell lymphoma. Lymphoid territories showed 80% positivity of Ki67. Neuropathologist concluded that the tumour was a myxopapillary ependymoma infiltrated by large B-cell lymphoma (Figure 2). CSF examination performed few weeks later showed that the CSF was highly invaded with malignant B-cell lymphoma.
After surgical treatment, patient was referred to haemato-oncologist to start systemic chemotherapy. Because of the unusual presentation and concomitant presence of the two histologically different tumours, original scheme of treatment was proposed. Patient underwent 2 cycles of high dose methotrexate with two intrathecal injections associated with rituximab, combined in second cycle with cyclophosphamide, vincristine and prednisone.
Pain relief was obtained after surgery. Functional rehabilitation permitted partial recovery of gait with walking frame. Patient was discharged to rehabilitation center 1 week after. A PET-TDM obtained a month later displayed intense hypermetabolism located between L4 and S1 level in spinal canal, potentially due to post-op inflammatory process or remnant of the tumours. At 2 years’ follow-up, patient remains highly disabled with paraparesis, urinary incontinence supplied by mechanical prosthesis. Furthermore, he is now considered in total remission according to the latest PET-scan showing no progression of lesions at the cervical, thoracic and lumbar spine and is now off treatment.
Discussion
This is the first report of concomitant spinal intradural lymphoma with multifocal myxopapillary ependymoma.
Even though there are published cases of myxopapillary ependymoma coexisting with other types of pathology, whether tumoral or not (3-5), our case emphasizes a unique finding at pathological examination of a large B-cell lymphoma proliferation in a myxopapillary ependymoma. The term of “tumour-to-tumour metastasis” has been previously described in CNS with most frequent recipient type identified as meningioma, followed by pituitary adenoma. The cases show mostly brain locations but rarely spinal and even less intradural. The most frequent donor types found in the literature are lung and breast carcinoma. Here, there is no evidence that one of the tumours was the host for the other as we have no prior imaging record. Full workup including whole-body CT-scan and 18-fluorodeoxyglucose (18-FDG) positron emission tomography (PET) found no evidence of extra spinal tumour neither brain ependymoma nor systemic lymphoma which suggests that it was a primary central nervous system lymphoma (PCNSL).
The term of “collision tumours” is also employed in such situation when two contiguous tumours develop in the same place as the case of a collision between prostate metastasis to the brain and an esophageal malignancy in the report of Dewan et al. (6). Their case is similar considering two different histologic tumours occurring in the same location but differs by their origin. The collision concerned non-CNS tumours metastasis in brain unlike our case which involves two CNS tumours. The mechanism remains unknown. Chemokine as IL-8 is known to be involved in chemo-attraction and seems to allow B cells to cross the blood-brain barrier which could explain the occurrence of CNS lymphomas. Modification of vascular wall in the ependymoma could have facilitated B cells to cross it and in this way, penetrate the prior tumour. However, whether this mechanism is involved in infiltrating an ependymoma remains uncertain.
Meningiomas have been identified as good host for tumours because of their rich vascularity making them susceptible to hematogenous spread, slow growth and slow metabolic rate and high collagen and immune content. An interaction between cell adhesion molecules has been advocated to explain the tendency of breast cancer to metastasize to meningioma (7). Such favorable soil is not described for ependymoma or lymphoma in the literature analyzing this phenomenon. However, we can speculate that adhesion molecules could be involved in the mechanism leading to our case.
Spreading from an extradural lymphoma to intradural space could have been suspected as it is most frequently encountered, but few cases of spinal intradural lymphoma have been published. Only 16 cases can be found reporting primary intradural extramedullary lymphoma in cauda equina location (8). In our case, no lymphoma was identified in systemic organ or in extradural space but only leptomeningeal dissemination. Such finding is unprecedented.Spinal cord ependymomas are preferentially myxopapillary subtype, involving cauda equina and filum terminale in most cases as a solitary lesion. Ependymomas represent 15% of spinal cord tumours and up to 60% of spinal cord gliomas. Another exceptional condition for this case is that we describe an extremely rare entity of multifocal ependymoma located in intradural extramedullary which was find at lumbar and cervical level. This presentation is exceptional with one case report of a grade II ependymoma published amongst the only 19 cases of intradural extramedullary ependymomas, excluding those comprising conus medullaris and filum terminale (9). Cooper et al. assumed that intradural extramedullary ependymomas originate from heterotopic glial cells (10). De Falco et al. reported a case of multifocal ependymoma in a 16-year-old man with a concomitant presentation of a thoracic spinal cord myxopapillary ependymoma with a second localization in filum terminale (11). The authors eliminated a disseminated ependymoma, a metastatic drop lesion or direct extension of a primary CNS tumour along the CSF and a spreading from the lumbosacral region to the cranial cavity.Although rare, this phenomenon (coexisting tumours within the same site) should be known by neurosurgeons because the possibility of collision tumours affects the treatment and the patient’s prognosis. In any case, the aim is to perform a complete excision of the tumour to allow maximum chances for the treatment to succeed. Adjuvant treatment remains challenging because of the uncommon presentation here. Oncologic therapies of two histologic lesions were associated, methotrexate and lymphoma protocol including rituximab, well discussed in multidisciplinary staff and reappraised depending on the oncologic response.
Conclusions
We report the first case of concomitant intradural extramedullary ependymoma infiltrated by primary CNS lymphoma, which is not only challenging for the neurosurgeon but also for all the therapists involved.
Acknowledgements
Thanks to Vincent Martin-Giardi for his precious help.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep 2010;10:240-7. [Crossref] [PubMed]
- Wolf NI, Harting I, Hartmann M, et al. Combination of caudal myxopapillary ependymoma and dermal sinus: a single shared embryologic lesion? Dev Med Child Neurol 2003;45:568-70. [Crossref] [PubMed]
- Tubbs RS, Kelly DR, Mroczek-Musulman EC, et al. Dwarfism, occult spinal dysraphism, and presacral myxopapillary ependymoma with an epidermoid cyst in a child. Acta Neurochir (Wien) 2005;147:299-302; discussion 302. [Crossref] [PubMed]
- Adamson DC, Cummings TJ, Friedman AH. Myxopapillary ependymoma and fatty filum in an adult with tethered cord syndrome: a shared embryological lesion? Case report. Neurosurgery 2005;57:E373; discussion E373.
- Dewan S, Alvarez VE, Donahue JE, et al. Intracranial collision metastases of prostate and esophageal carcinoma. J Neurooncol 2009;95:147-50. [Crossref] [PubMed]
- Aghi M, Kiehl TR, Brisman JL. Breast adenocarcinoma metastatic to epidural cervical spine meningioma: case report and review of the literature. J Neurooncol 2005;75:149-55. [Crossref] [PubMed]
- Nakashima H, Imagama S, Ito Z, et al. Primary cauda equina lymphoma: case report and literature review. Nagoya J Med Sci 2014;76:349-54. [PubMed]
- Iunes EA, Stávale JN, de Cássia Caldas Pessoa R, et al. Multifocal intradural extramedullary ependymoma. Case report. J Neurosurg Spine 2011;14:65-70. [Crossref] [PubMed]
- Cooper IS, Craig WM, Kernohan JW. Tumors of the spinal cord; primary extramedullary gliomas. Surg Gynecol Obstet 1951;92:183-90. [PubMed]
- De Falco R, Scarano E, Di Celmo D, et al. Concomitant localization of a myxopapillary ependymoma at the middle thoracic part of the spinal cord and at the distal part of the filum terminale. Case report. J Neurosurg Sci 2008;52:87-91. [PubMed]


