
Clinical and radiographic analysis of expandable versus static lateral lumbar interbody fusion devices with two-year follow-up
Introduction
Minimally invasive lateral lumbar interbody fusion (LLIF) has become an increasingly popular option for the treatment of patients with degenerative disc disease (DDD) and related spinal disorders requiring surgical intervention. This surgical approach allows for the indirect decompression of neural elements and direct visualization of the intervertebral disc space for placement of a large-footprint intervertebral spacer. Furthermore, LLIF is associated with lower rates of complications that are reported for open anterior and posterior approaches, including damage to anterior and posterior longitudinal ligaments, nerve root injury, bony resection, and incidental durotomy (1,2). However, postoperative transient thigh pain due to dissection of the psoas muscle is a known complication from LLIF that is unique to the approach (3).
Early reports on the development of interbody spacers for spinal arthrodesis, and interest in their use, date back several decades (4,5). Since then, interbody spacers have been widely used in the management of spinal pathologies, and favorable outcomes have been reported following their use in anterior, posterior, and transforaminal lumbar interbody fusion procedures (6-9). The majority of clinical outcome studies have focused on static interbody spacers, but expandable devices have become available in recent years. Expandable spacers are designed to be inserted at a minimized profile and expanded in situ for decreased trialing and iatrogenic endplate disruption secondary to impaction, in comparison to static devices. The potential clinical advantages of expandable devices include less neural retraction, decreased endplate damage, and less implant subsidence and/or migration (10-12).
To the authors’ knowledge, no clinical study to date has compared static and expandable spacers in the treatment of patients with symptomatic lumbar pathology following LLIF. Therefore, the aims of this study were to compare clinical and radiographic outcomes after LLIF using static and expandable interbody spacers.
Methods
Patient population
This multicenter clinical study included a total of 56 patients (63 operative levels) with objective evidence of DDD at one or two contiguous levels at L2–S1. Twenty-nine patients underwent minimally invasive LLIF with a static spacer (TransContinental®, Globus Medical, Inc., Audubon, PA), and 27 with an expandable spacer (CALIBER®-L, Globus Medical, Inc.) (Figures 1,2). All procedures were combined with supplemental transpedicular posterior fixation, and all patients reached 24-month follow-up. Patients who satisfied the inclusion/exclusion criteria were included (Table 1) and provided informed consent to participate in the study. All participating institutions received Institutional Review Board approval.


Full table
Surgical technique
Patients were placed in the left lateral decubitus position, and under fluoroscopic guidance, a laterally centered oblique incision was made over the involved disc segment. Blunt dissection was performed under direct visualization through subcutaneous tissue, external and internal oblique muscles, and transversus abdominis. The retroperitoneal fat was mobilized anteriorly—exposing the underlying psoas muscle, which was dissected, in line with its fibers, down to the operative intervertebral disc level. Dilators were placed and the retractor positioned and secured to the table-mounted arm. A lateral fluoroscopic image was then obtained to confirm appropriate level, placement, and rotation. An annulotomy was then performed, and sequential spacers were placed under anteroposterior imaging to allow for gradual distraction of the disc space.
A static or expandable spacer of appropriate size was selected, packed with appropriate autogenous bone graft, and implanted laterally across the disc space under fluoroscopic imaging. The expandable spacer was expanded to the desired height and back-filled with autogenous bone graft (Figures 3,4).
The expandable interbody spacer used in this study is manufactured from titanium alloy and radiolucent polyether-ether-ketone (PEEK) polymer. The device is inserted at a contracted height and expanded in situ once correctly positioned within the intervertebral space, offering continuous expansion for optimal endplate-to-endplate contact (Figure 5). The static interbody spacer is composed of radiolucent PEEK polymer and includes a self-distracting leading edge for implant insertion.
Outcomes assessment
Demographic and perioperative data were recorded. Patient self-assessment questionnaires, including the visual analog scale (VAS) to quantify low back pain (0–10 mm scale) and Oswestry Disability Index (ODI) to gauge functional disability, were evaluated preoperatively and at 6 weeks, and 3, 6, 12, and 24 months postoperatively. Radiographic parameters, including intervertebral fusion, implant subsidence, implant migration, intervertebral and neuroforaminal heights, and segmental lordosis were assessed preoperatively, and at 6 weeks and 3, 6, 12, and 24 months postoperatively. Radiographic fusion was assessed according to the five-point scale of Brantigan and Steffee: (I) obvious radiographic pseudoarthrosis; (II) probable radiographic pseudoarthrosis; (III) radiographic status uncertain; (IV) probable radiographic fusion; and (V) radiographic fusion. Fusion for this clinical study was defined as a grade of 4 or 5 according to this criterion (13). Postoperative implant subsidence was defined as a reduction in intervertebral disc height greater than 2 mm in comparison with 6-week postoperative measurements. Intervertebral disc height was measured at the middle of the endplates immediately above and below the referenced index levels on the lateral plane. Neuroforaminal height was measured as the distance from the inferior pedicle wall of the level above to the superior pedicle wall of the level below. The segmental lordosis was measured from the superior endplate of the cephalad vertebral body to the inferior endplate of the caudal vertebral body.
Statistical methods
Statistical analysis was performed with SPSS® v20.0.0 software for Windows (IBM Corp., Armonk, NY, USA). The Wilcoxon signed rank test and a paired sample t-test were used to calculate changes in ordinal and interval variables from preoperative to each postoperative time interval. The Wilcoxon Mann-Whitney test for ordinal variables and an independent sample t-test for interval variables were used for comparison between groups. Additionally, a Chi-square test was performed to assess differences in categorical variables between groups. Statistical significance was indicated at P<0.05.
Results
Patient demographic and operative data
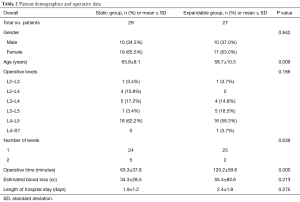
The patient cohort comprised 36 females and 20 males with a mean age of 62.3±10.3 years (range, 33–81 years). Single-level fusion was performed in 87.5% (49/56) of patients, and 12.5% (7/56) underwent two-level fusion. Surgery was most common at the L4–L5 intervertebral level for both static and expandable groups (60.7%). Treatment groups had no significant differences in terms of gender, operative level, number of levels, estimated blood loss, or length of hospital stay (P>0.05). However, statistical differences were observed in age (65.8±9.1 in the static group versus 58.7±10.5 in the expandable group, P=0.009) and operative time between static (63.3±37.8 minutes) and expandable groups (120.2±59.6 minutes) (P=0.000) (Table 2). The observed difference in operative time was considered secondary to patients with static spacers having unilateral posterior stabilization, whereas nearly all patients with expandable spacers received bilateral posterior stabilization (23/27, 85%), requiring repositioning to prone position.
Full table
Clinical outcomes
Patient groups reported similar improvements in VAS back pain and ODI scores. Mean VAS back pain scores in the static group improved significantly from 6.9±2.3 preoperatively to 3.9±2.8 at 24 months postoperatively, and in the expandable group from 6.9±2.6 preoperatively to 3.8±3.6 at 24 months postoperatively (P=0.01). ODI scores also improved significantly in the static group (46.6±19.4 preoperatively to 26.2±19.8 at 24 months postoperatively) and in the expandable group (49.6±16.8 preoperatively to 30.3±25.4 at 24 months postoperatively) (Table 3). Postoperative VAS and ODI scores across time intervals showed no significant differences between groups at any time interval (P>0.05).
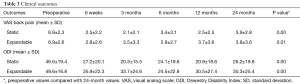
Full table
Radiographic outcomes
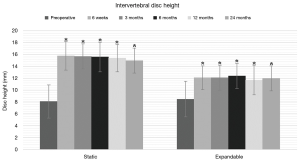
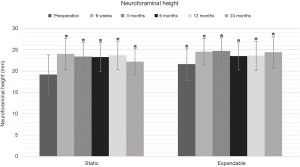
Preoperative intervertebral and neuroforaminal heights increased significantly in both groups at 24-month follow-up. In the static group, intervertebral disc height increased from 8.1±2.8 mm preoperatively to 15.0±2.1 mm postoperatively, and neuroforaminal height from 19.2±4.6 mm preoperatively to 22.2±3.1 mm at 24-month follow-up (P<0.01). In the expandable group, corresponding parameters increased from 8.5±3.0 mm preoperatively to 12.0±2.1 mm postoperatively, and from 21.6±3.8 mm preoperatively to 24.4±3.7 mm postoperatively (P<0.01) (Figures 6,7). Significant differences in intervertebral disc height were observed between groups at 6 weeks through 12 months (P<0.05). The comparison of neuroforaminal height between groups showed no statistical significance (P>0.05). Moreover, segmental lordosis showed no significant changes at any follow-up interval, and no significant differences between groups (P>0.05). Solid fusion was observed in all patients by 24-month follow-up.
Implant-related observations
The occurrence of radiographic implant subsidence was significantly different between groups with a rate of 16.1% (5/31 levels) in the static group and none in the expandable group (P<0.01) (Figure 8). Neither group exhibited evidence of implant migration at any operative level and no cases of surgical revision at the index or adjacent levels were reported.
Discussion
LLIF is commonly performed to manage patients with lumbar pathology. The lateral technique circumvents complications typically associated with conventional posterior approaches to the lumbar spine, including musculoligamentous and neurologic complications (1,14). Among the many advantages of the lateral approach is the ability to insert a larger-footprint implant in the intervertebral space, allowing the implant to span the apophyseal ring and increase contact with the peripheral cortices. Although several authors have reported on the advantages of LLIF (15,16), no clinical studies to date have examined the clinical and radiographic outcomes of static versus expandable lateral interbody spacers in an LLIF application.
Static interbody spacers have long been considered the gold standard for the treatment of patients with degenerative disorders of the spine. Although LLIF procedures performed with static interbody spacers have produced favorable clinical outcomes (17-19), excessive spacer trialing and forceful impaction may lead to iatrogenic endplate damage, which may induce complications such as spacer migration, subsidence, breakage, and pseudoarthrosis (10,11,14,16). To address these issues, expandable interbody spacers were designed for insertion at a low profile, and expanded in situ to mitigate iatrogenic endplate damage secondary to implant trialing and impaction. Although the use of both static and expandable spacers in this study led to significant improvement in clinical and radiographic outcomes and high fusion rates, subsidence was of greater concern in the static spacer group.
Implant subsidence was significantly higher in the static group (16.1%) than the expandable group (0%). Low-grade subsidence (<2 mm) of an interbody spacer is an expected postoperative event (20). However, high-grade subsidence (≥2 mm) can have adverse clinical consequences such as loss of disc height and indirect neural decompression, increased stenosis, resurgence of symptomatic pathology, nonunion, sagittal imbalance, and reoperation (16,20,21). Factors believed to be associated with interbody implant subsidence include stand-alone implant placement, forceful impaction (21), over-distraction of the intervertebral space (22-25), and the use of narrow spacers (20,26). The authors of the current study consider that the impaction force and over-distraction necessary to insert a static implant may have contributed to higher rate of subsidence. The insertion of an expandable spacer requires less impaction due to its reduced initial profile and controlled expansion when attaining optimal disc height. Importantly, all instances of subsidence were asymptomatic and all levels reached successful fusion 24 months postoperatively. Of the implants that subsided, most spacers (2/5) subsided into the superior endplate, one subsided into the inferior endplate, and two into both inferior and superior endplates, which is consistent with the literature that states that the superior endplate is 40% weaker than the inferior (14,26,27).
A limitation of this study is the absence of computed tomography (CT) scans for assessment of fusion, as CT scans are not routinely performed as part of the follow-up regimen. Furthermore, longer follow-up is often required to fully assess solid fusion and complications such as adjacent level disease. The findings of this study suggest that the use of both static and expandable interbody spacers in LLIF results in improvement in patient pain and disability, an early return to function at 6 weeks that is maintained through 12 months, increased disc height and neuroforaminal height, and reliably high fusion rates. Asymptomatic subsidence was of greater concern in the static group. Future studies should focus on longer-term follow-up and the enrollment of larger patient cohorts to further examine differences between static and expandable spacers.
Acknowledgements
The authors acknowledge that funding for this project was provided by the Musculoskeletal Education and Research Center (MERC), a Division of Globus Medical, Inc.
Footnote
Conflicts of Interest: Dr. Frisch is a consultant for and receives royalties from Globus Medical. Dr. O’Brien is a consultant for Globus Medical, RTI Surgical, DePuy Synthes, and 4WEB Medical; has stocks with RTI Surgical, Alphatec Spine, 4WEB Medical; receives royalties from Globus Medical, NuVasive Inc., and RTI Surgical; and receives research support from the National Science Foundation, NuVasive Inc., and RTI Surgical. DM Brooks, IY Luna and G Joshua are salaried employees of Globus Medical.
Ethical Statement: All participating institutions received Institutional Review Board approval. Patients who satisfied the inclusion/exclusion criteria were included and provided informed consent to participate in the study.
References
- Hosono N, Namekata M, Makino T, et al. Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: analysis of risk factors. J Neurosurg Spine 2008;9:403-7. [Crossref] [PubMed]
- Garg J, Woo K, Hirsch J, et al. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg 2010;51:946-50. [Crossref] [PubMed]
- Pumberger M, Hughes AP, Huang RR, et al. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J 2012;21:1192-9. [Crossref] [PubMed]
- Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics 1988;11:931-4. [PubMed]
- McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am 1999;81:859-80. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012;2012:246989. [PubMed]
- Sharma AK, Kepler CK, Girardi FP, et al. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech 2011;24:242-50. [Crossref] [PubMed]
- Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976) 2010;35:S302-11. [Crossref] [PubMed]
- Phillips FM, Isaacs RE, Rodgers WB, et al. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976) 2013;38:1853-61. [Crossref] [PubMed]
- Aoki Y, Yamagata M, Nakajima F, et al. Examining risk factors for posterior migration of fusion cages following transforaminal lumbar interbody fusion: a possible limitation of unilateral pedicle screw fixation. J Neurosurg Spine 2010;13:381-7. [Crossref] [PubMed]
- Chen L, Yang H, Tang T. Cage migration in spondylolisthesis treated with posterior lumbar interbody fusion using BAK cages. Spine (Phila Pa 1976) 2005;30:2171-5. [Crossref] [PubMed]
- Elias WJ, Simmons NE, Kaptain GJ, et al. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg 2000;93:45-52. [PubMed]
- Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion: two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 1993;18:2106-7. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Maintenance of segmental lordosis and disc height in standalone and instrumented extreme lateral interbody fusion (XLIF). Clin Spine Surg 2017;30:E90-E98. [Crossref] [PubMed]
- Kotwal S, Kawaguchi S, Lebl D, et al. Minimally invasive lateral lumbar interbody fusion: clinical and radiographic outcome at a minimum 2-year follow-up. J Spinal Disord Tech 2015;28:119-25. [Crossref] [PubMed]
- Kim SJ, Lee YS, Kim YB, et al. Clinical and radiological outcomes of a new cage for direct lateral lumbar interbody fusion. Korean J Spine 2014;11:145-51. [Crossref] [PubMed]
- McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976) 2005;30:S60-5. [Crossref] [PubMed]
- Shunwu F, Xing Z, Fengdong Z, et al. Minimally invasive transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases. Spine (Phila Pa 1976) 2010;35:1615-20. [Crossref] [PubMed]
- Rouben D, Casnellie M, Ferguson M. Long-term durability of minimal invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech 2011;24:288-96. [Crossref] [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8. [Crossref] [PubMed]
- Kwon AJ, Hunter WD, Moldavsky M, et al. Indirect decompression and vertebral body endplate strength after lateral interbody spacer impaction: cadaveric and foam-block models. J Neurosurg Spine 2016;24:727-33. [Crossref] [PubMed]
- Truumees E, Demetropoulos CK, Yang KH, et al. Effects of disc height and distractive forces on graft compression in an anterior cervical discectomy model. Spine (Phila Pa 1976) 2002;27:2441-5. [Crossref] [PubMed]
- Alimi M, Shin B, Macielak M, et al. Expandable polyaryl-ether-ether-ketone spacers for interbody distraction in the lumbar spine. Global Spine J 2015;5:169-78. [Crossref] [PubMed]
- Gonzalez-Blohm SA, Doulgeris JJ, Aghayev K, et al. In vitro evaluation of a lateral expandable cage and its comparison with a static device for lumbar interbody fusion: a biomechanical investigation. J Neurosurg Spine 2014;20:387-95. [Crossref] [PubMed]
- Tohmeh AG, Khorsand D, Watson B, et al. Radiographical and clinical evaluation of extreme lateral interbody fusion: effects of cage size and instrumentation type with a minimum of 1-year follow-up. Spine (Phila Pa 1976) 2014;39:E1582-E1591. [Crossref] [PubMed]
- Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37:1268-73. [Crossref] [PubMed]
- Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976) 2001;26:889-96. [Crossref] [PubMed]



