
Do intra-operative neurophysiological changes predict functional outcome following decompressive surgery for lumbar spinal stenosis? A prospective study
Introduction
Symptomatic lumbar spinal stenosis (LSS) with neurogenic intermittent claudication (NIC) can be treated either conservatively or surgically in particular if symptoms persist and are significantly impairing function (1,2). Decompressive surgery is regarded as the standard surgical approach for symptomatic LSS who have failed conservative treatment. Even though this is a relatively safe procedure neurological, complications following this type of surgery have been described (3,4) in particular as this population is quite old and likely have multiple co-morbidities. The role of intraoperative neurophysiological monitoring (IONM) in spinal surgery is not completely established and remains controversial (5) but is increasingly used in particular in deformity surgery (6-9). Neurophysiological changes have been documented during lumbar decompression (10,11) and its value in detecting neurological damage with a high sensitivity and specificity have been documented (12). The relation of neurophysiological changes during lumbar decompression and postoperative functional outcome is not well established.
To the authors’ knowledge neurophysiological improvement and its relation to early and long-term post-operative clinical outcome have been incompletely studied and the authors believe this is the first study to address this clinical question.
Therefore, our aim was to study the relation between immediate intraoperative neurophysiological changes after surgical decompression and functional clinical outcome using the Zurich Claudication Questionnaire (ZCQ) self-assessment score in a series of patients with LSS undergoing surgery.
Methods
Human research ethics approval was obtained. We prospectively collected clinical data from a cohort of 24 consecutive patients undergoing decompression during a 28 months period (between October 2010 and February 2012) at a single institution and by a single surgeon.
Preoperatively all patients underwent magnetic resonance imaging (MRI) examinations of their lumbar spine pre-operatively as part of the standard work up protocol. All patients had central stenosis with or without lateral recess stenosis. No patient with cord compression was included even though four cases presented with L1–2 involvement but at cauda equina level. All patients had failed conservative treatment before consenting for surgery.
Anesthetic technique
Anesthetic induction and maintenance of anesthesia was performed by a total intravenous anesthesia using Sufentanil and Propofol (13,14). Only non-depolarizing muscle relaxants were applied for intubation. Volatile anesthetics were not used during the procedure. Wake up tests were not performed.
Surgical technique
The patients were positioned prone on a Montreal mattress. Patients underwent posterior lumbar decompression of all levels that showed morphological evidence of stenosis on preoperative MRI (grades C&D) (15).
After exposing the posterior elements, our standard surgical procedure consists in distracting the concerned levels by an interlaminar spreader introduced between the spinous processes. This step allows better approach to the interlaminar space, especially if osteophytes are occulting the ligamentum flavum. To complete the decompression medial facet osteophytes were removed with a high-speed burr, followed by the excision of the ligamentum flavum associated to a bilateral laminotomies until the dural sac and nerve roots were completely identified and freed.
IONM technique
Intraoperative trans-cranial motor evoked potentials (tcMEPs) were measured prior to decompression (baseline) in prone position. Depending on the treated level the tcMEPs were acquired from two to four lower limb muscles (bilateral tibialis anterior/abductor hallucis muscles). The reference control value was the response of one upper limb muscle (1st interosseous muscle of the hand). Using two corkscrew electrodes, localized at C1 C2, a transcranial electrical stimulation was triggered at the standard derivations by a 500 Hz train of 5 to 7 1-ms biphasic impulses. Stimulation between 50 to 150 volts was adequate to provoke a consistent motor response. The main outcome measure of tcMEP recording was the relative change of the area under the curve (AUC) of the normalized (to the hand interosseous response) motor response as previously described (16) before and immediately after full decompression. The last measurement coincided with the end of the surgical procedure, shortly before closure. A 20% improvement has been selected as significant for the purpose of this study (17). This neurophysiological outcome measure was subsequently related to the ZCQ scores (at baseline, early and late follow up time points as described below). Free running electromyography (EMG) analysis was not performed routinely in our study although it has been used in some cases on demand but without prospectively recording results since this was outside our study protocol. In addition, even though we routinely monitored somatosensory evoked potentials (SSEPs) (through the posterior tibial nerve) we did not use that data since it concerned mainly one nerve root (S1).
Follow-up
Postoperatively patients were followed by at regular intervals by the surgical team. Latest self-assessment questionnaire was obtained either during that last visit or by postal correspondence.
Complete early and late follow up data was available in 18 of those cases at an average of 8 months (early follow-up) and at 29 months (long term follow-up).
Functional evaluation
The ZCQ self-assessment score served as primary functional outcome measure. It consists in a three item score measuring symptom severity and physical function, as well as patient’s satisfaction following surgery. The 0.5 scale points change (i.e., relative change divided by baseline must be at least greater than 0.5) has been considered as significant for this study as previously reported (18,19). Patients completed the ZCQ before surgery (baseline), at an average of 8 (range, 3–12) and of 29 (range, 21–37) months following surgery (short and long term outcome, respectively).
Statistical analysis
Fisher’s exact test and the Linear Pearson Correlation test were used as appropriate. Little number of patients made dichotomizing of data necessary.
Results
There were 24 patients included in the prospective study with mean age of 69 years (range, 51–84 years). Male:female ratio was 0.8 in this cohort. Eighteen patients had long-term follow-up. Ten patients had single level involvement and eight had multilevel surgery (Table 1). There were no neurological injuries following surgery.
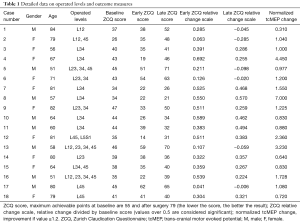
Full table
The average baseline ZCQ score was 72% (range, 47–85%). At early follow-up an average score of 46% (range, 24–78%) was achieved with an average change of 26% (range, 3–54%). There was no significant association between change in ZCQ score at early follow-up with gender (P=0.389) or age of the patient (P=0.627). At late follow-up the average ZCQ-score was of 57% (range, 27–90%) with an average absolute change from baseline of 15.5 (range, 14–40). There was no significant association between change in ZCQ score at late follow-up with gender (P=0.478) or age of the patient (P=0.834). There was no correlation between any of the demographic variables and improvement in ZCQ.
At early follow-up all patients showed improvement in the absolute result of ZCQ. From those, seven did so in a significant way (relative point scale score >0.5). At latest follow-up, only four patients had still a significant improvement of their ZCQ score compared to the baseline outcome (relative point scale score >0.5).
Eight patients showed an intra-operative improvement of their tcMEP in excess of 20%, while three improved less than 20%. Seven patients did not show any tcMEP improvement at all at the end of the decompression.
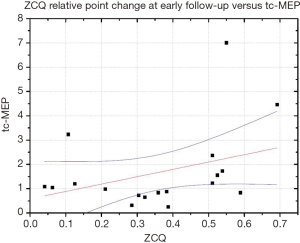
We found a moderate positive correlation (R=0.38), between tcMEP changes and ZCQ relative point score at early follow-up (Figure 1).
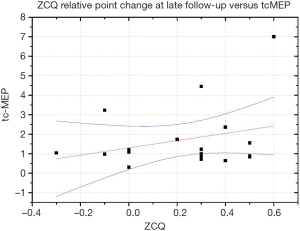
At latest follow-up nevertheless only a very fair correlation (R=0.11) was found between tcMEP and ZCQ changes (Figure 2).
Dichotomizing the data using a 50% improvement for ZCQ and 20% for tcMEPs as cut-off points showed a statistically significant relation between tcMEP improvement and better functional outcome at early follow-up (P=0.013) (Table 2) disappearing at 24 months (P=1) (Table 3).

Full table

Full table
Discussion
In this study we found that tcMEP improvement was related to a better functional outcome but only in the early follow-up. This improvement was found to between equivalent amongst males vs. females and similar across the age range studied.
The constantly increasing number of surgical procedures demands thorough vigilance towards integrity of neural structures (20). In order to receive real-time feedback, neurophysiological assessments during surgery were introduced and have developed into a useful tool (7,10,21) IONM has clearly been shown to be effective in spinal cord tumours (22). Its use is nevertheless not widely accepted. Sharan et al. couldn’t find any evidence in the literature that IONM can help in preventing nerve root injuries in the context of pedicle instrumentation (23). Similarly, not all neurological incidents had been recognized by IONM in a study by Alemo et al. (5). There is not always a distinction in literature reviews between the different modalities in particular between SSEPs and motor evoked potentials (MEPs) which differ in their prognostic value with SSEPs being regarded as less sensitive (24).
Little is known so far about the possible positive effect of surgical decompression procedures to the electrophysiological response and functional outcome.
Most recently the IONM, more precisely the evoked potentials (EPs) in general, are gaining importance as so called, biomarkers (25).
The full validation of IONM is in process and controlled trials are required to confirm its role; a very difficult task, because patients will not except to relinquish the potential benefit of this tool (25).
Studying the specificity and sensitivity of IONM is beyond the scope of our research. We aimed to identify any relation between intraoperative tcMEP changes and functional outcome, something that has to our knowledge been studied only incompletely.
Indeed Voulgaris et al. compared the IONM responses to the visual analog scale (VAS) score at 12 months postoperatively and found a greater improvement in the VAS score for patients demonstrating significant tcMEP improvement (26). VAS score is nevertheless not disease specific and ZCQ had not been studied.
Our study is therefore the first one to compare the IONM with a disease specific functional outcome score. The present study shows that immediate neurophysiological response in IONM after decompressive surgery for LSS is correlated with a positive effect on the clinical outcome after an average of 8 months of follow up. At late follow-up of more than 28 months after surgery the beneficial effect of decompression surgery declines gently and no significant correlation could be found between the tcMEPs response improvement and ZCQ score. The outcome worsening at long term is commonly observed in other studies on surgical outcomes following decompression (27).
The present study is limited by several constraints. Our study is a small case series, but it does give a neurophysiological account of the immediate changes observed during decompressive surgery. We did observe though that the lack of tcMEP improvement was somehow in relation to a lesser functional improvement whatever the origin of this poor tcMEP response might have been. Since we used only intra-operative tcMEPs to compare with functional scores, we were not able to describe the neurophysiological response at late follow-up. Future research should focus on late neurophysiological changes in a larger cohort of patients. In addition the clinical outcome score is a self administered questionnaire and not an objective measure although this type of subjective outcome is widely used in spinal surgery.
Our findings suggest that intra-operative neurophysiological improvement during decompressive surgery may predict clinical outcome at 6 months following surgery. Nevertheless, as it has been observed with other spinal procedures, the initial improvement in functional outcome diminishes with the passage of time making the relation between function and neurophysiological changes less meaningful. Initial neurophysiological changes could be useful in predicting short-term failures. The small number of cases presented in this paper makes it mandatory to apply caution in the interpretation of our results. Further research with a greater number of cases and a more homogeneous population would be necessary before drawing definitive conclusions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the local ethical committee—Commission d’Ethique (Bugnon 21, 1005 Lausanne, Switzerland).
References
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008;358:794-810. [Crossref] [PubMed]
- Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine (Phila Pa 1976) 2011;36:E1335-51. [Crossref] [PubMed]
- Lad SP, Babu R, Ugiliweneza B, et al. Surgery for spinal stenosis: long-term reoperation rates, health care cost, and impact of instrumentation. Spine (Phila Pa 1976) 2014;39:978-87. [Crossref] [PubMed]
- Jakola AS, Sørlie A, Gulati S, et al. Clinical outcomes and safety assessment in elderly patients undergoing decompressive laminectomy for lumbar spinal stenosis: a prospective study. BMC Surg 2010;10:34. [Crossref] [PubMed]
- Alemo S, Sayadipour A. Role of intraoperative neurophysiologic monitoring in lumbosacral spine fusion and instrumentation: A retrospective study. World Neurosurg 2010;73:72-6; discussion e7.
- Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine 2005;2:725-32. [Crossref] [PubMed]
- Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am 2007;89:2440-9. [PubMed]
- Pastorelli F, Di Silvestre M, Plasmati R, et al. The prevention of neural complications in the surgical treatment of scoliosis: the role of the neurophysiological intraoperative monitoring. Eur Spine J 2011;20 Suppl 1:S105-14. [Crossref] [PubMed]
- Flynn JM, Sakai DS. Improving safety in spinal deformity surgery: Advances in navigation and neurologic monitoring. Eur Spine J 2013;22 Suppl 2:S131-7. [Crossref] [PubMed]
- Lall RR, Lall RR, Hauptman JS, et al. Intraoperative neurophysiological monitoring in spine surgery: indications, efficacy, and role of the preoperative checklist. Neurosurg Focus 2012;33:E10. [Crossref] [PubMed]
- Sutter M, Eggspuehler A, Muller A, et al. Multimodal intraoperative monitoring: An overview and proposal of methodology based on 1,017 cases. Eur Spine J 2007;16 Suppl 2:S153-61. [Crossref] [PubMed]
- Sutter MA, Eggspuehler A, Grob D, et al. Multimodal intraoperative monitoring (MIOM) during 409 lumbosacral surgical procedures in 409 patients. Eur Spine J 2007;16 Suppl 2:S221-8. [Crossref] [PubMed]
- DiCindio S, Theroux M, Shah S, et al. Multimodality monitoring of transcranial electric motor and somatosensory-evoked potentials during surgical correction of spinal deformity in patients with cerebral palsy and other neuromuscular disorders. Spine (Phila Pa 1976) 2003;28:1851-5; discussion 1855-6.
- Wang AC, Than KD, Etame AB, et al. Impact of anesthesia on transcranial electric motor evoked potential monitoring during spine surgery: a review of the literature. Neurosurg Focus 2009;27:E7. [Crossref] [PubMed]
- Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010;35:1919-24. [Crossref] [PubMed]
- Macdonald DB. Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput 2006;20:347-77. [Crossref] [PubMed]
- MacDonald DB, Al Zayed Z, Khoudeir I, et al. Monitoring scoliosis surgery with combined multiple pulse transcranial electric motor and cortical somatosensory-evoked potentials from the lower and upper extremities. Spine (Phila Pa 1976) 2003;28:194-203. [Crossref] [PubMed]
- Stucki G, Daltroy LD, Liang MH, et al. Measurement Properties of a Self-Administered Outcome Measure in Lumbar Spinal Stenosis. Spine (Phila Pa 1976) 1996;21:796-803. [Crossref] [PubMed]
- Stucki G, Liangi MH, Fossel AH, et al. Relative responsiveness of condition-specific and generic health status measures in degenerative lumbar spinal stenosis. J Clin Epidemiol 1995;48:1369-78. [Crossref] [PubMed]
- Gonzalez AA, Jeyanandarajan D, Hansen C, et al. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus 2009;27:E6. [Crossref] [PubMed]
- Sutter M, Deletis V, Dvorak J, et al. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J 2007;16 Suppl 2:S232-7. [Crossref] [PubMed]
- Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery 2006;58:1129-43; discussion 1129-43. [Crossref] [PubMed]
- Sharan A, Groff MW, Dailey AT, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine 2014;21:102-5. [Crossref] [PubMed]
- Macdonald DB, Skinner S, Shils J, et al. Intraoperative motor evoked potential monitoring - a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol 2013;124:2291-316. [Crossref] [PubMed]
- Holdefer RN, MacDonald DB, Skinner SA. Somatosensory and motor evoked potentials as biomarkers for post-operative neurological status. Clin Neurophysiol 2015;126:857-65. [Crossref] [PubMed]
- Voulgaris S, Karagiorgiadis D, Alexiou GA, et al. Continuous intraoperative electromyographic and transcranial motor evoked potential recordings in spinal stenosis surgery. J Clin Neurosci 2010;17:274-6. [Crossref] [PubMed]
- Lurie JD, Tosteson TD, Tosteson A, et al. Long-term outcomes of lumbar spinal stenosis: eight-year results of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976) 2015;40:63-76. [Crossref] [PubMed]


