
Permanent implantation of antibiotic cement over exposed instrumentation eradicates deep spinal infection
Introduction
Implant associated infection is a catastrophic and difficult to manage complication following spine surgery. Tuberculosis manifestations in the spine were first described hundreds of years ago, and since that time, the morbidity of spine infections have been well documented in the medical literature (1). Unfortunately, infections involving spinal instrumentation are associated with even greater rates of disability (2). The aging population in the United States and an increasing amount of degenerative spinal conditions has led to an increased demand for surgical reconstructions (3). With recent technological advancements and subsequently expanded surgical indications for instrumented procedures, the number of implant-associated infections may rise correspondingly. The successful management of this potentially devastating complication will become even more critical.
The incidence of infection following posterolateral instrumented fusion in the thoracolumbar spine is between 2–6% (4-7) and has been reported at over 15% in deformity cases (8). The rate of infection is multifactorial—based on patient and surgical factors, such as type of procedure, length of surgery, patient comorbidities and nutritional status, and various other factors. Spinal infections without instrumentation are often successfully treated with antimicrobial therapy alone; however, the presence of instrumentation provides an optimized environment for bacterial colonization and growth (9). When suspected, the management of a deep spinal infection must be aggressive with parenteral, culture-directed antibiotics as well as surgical debridement and irrigation.
Surgical site infection (SSI) is one of the most common nosocomial infections affecting post-operative patients with significant social and economic consequences (10). Post-operative spinal infections with instrumentation are particularly subject to an increased risk of pseudarthrosis and neurologic compromise, total hospital cost, and overall patient mortality (11). Unlike the gold standard, two-stage revision in North American hip and knee arthroplasty, there exists no standardized, accepted protocol for the management of deep SSI with instrumentation (12). Because removal of hardware in an unstable, instrumented spine can result in serious neurologic sequelae, retention of instrumentation with elimination of bacterial colonization on implants remains the goal.
The major objective of this study is to present a novel, efficacious protocol for the treatment of implant-associated spine infections, while minimizing the need for multiple debridements and hardware removal and optimizing infection eradication. Although Chen and Lee previously published a related case report describing the implantation of a permanent antibiotic cement strut for the treatment of cervical pyogenic spondylitis, this is the first case series in the English literature utilizing our novel treatment protocol (13).
Methods
Study population
This study was conducted at a single 1,200 bed academic, university-based medical center. All procedures were performed by a single fellowship trained orthopaedic spine surgeon. All aspects of this study were reviewed and approved by Columbia University’s institutional review board. Using Current Procedural Terminology (CPT) codes, institutional medical records were queried to identify all posterior spinal procedures performed by the aforementioned surgeon from the period of October 2008 to June 2014. These included all cervical, thoracic, and lumbar posterior spinal procedures for the treatment of both degenerative and deformity pathologies. The search identified 414 total patients who underwent 451 posterior spinal procedures. Each chart was reviewed on a case-by-case basis. Of the 414 patients included in our population, 34 were identified as having post-operative SSI (8.2%): ten patients with deep (2.4%) and 24 with superficial (5.8%). Superficial and deep infections were evaluated with advanced imaging in all cases: computed tomography and/or magnetic resonance imaging.
All 34 cases of SSI were identified based on CDC/NHSN criteria (14). All infections were classified as early post-operative SSI based on their occurrence within 1 year of instrumentation. Deep infections were defined as those which involved the deep soft tissue, muscle, and fascia, in contrast to superficial infections involving the skin and subcutaneous fat. All index procedures were performed with standard sterile technique, use of titanium and cobalt alloy implants, and placement of intrawound vancomycin powder (1 g). Exclusion criteria for the SSI subgroup included: superficial SSI and patients with less than 36 months of follow-up. The study population, therefore, consisted of ten patients with early post-operative infection who were subjected to our novel treatment protocol.
Surgical technique
When clinical concern for early post-operative deep SSI is present, it is the preference of the senior author to first begin with a standard irrigation and debridement procedure of the posterior spinal wound. Allograft and autograft are removed from the wound and all hardware is retained. The wound is debrided and subsequently copiously irrigated with 30 to 60 liters of normal saline. Although relatively arbitrary in the specific amount, the copious quantity of saline irrigation promotes bacterial dilution. A combination of bone graft substitutes is used to enhance fusion, including allograft cancellous chips, demineralized bone matrix, and minimally processed corticocancellous allograft. Antibiotic impregnated cement is prepared by combining a 40 g bag of cement with 2 g of vancomycin and 3.6 g of tobramycin powder. This preparation is then shaped into a cylinder spanning the length of the instrumentation and molded directly onto the exposed hardware bilaterally (Figure 1). If more cement is needed to cover the instrumentation, a second 40 g bag of cement is prepared with antibiotics in a similar manner.
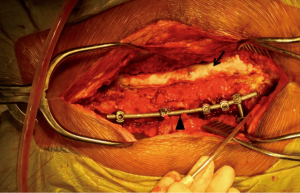
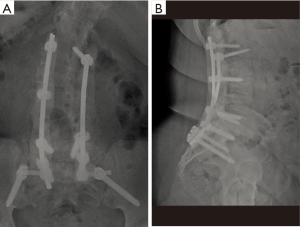
The wound is primarily closed and a retention suture technique is utilized if there is any tension on the wound edges. Hemovac drains are placed in the deep wound below the level of the fascia and remain on self-suction for at least 72 hours. After a minimum of 72 hours, the drains are discontinued when the output is less than 10 cc per 12-hour shift. At no point are wound or incisional negative pressure dressings utilized. Figure 2 is a postoperative anteroposterior (AP) and lateral radiograph demonstrating antibiotic cement overlying the instrumentation construct.
The importance of consultation from the infectious disease department cannot be overemphasized. Throughout the entire course, the patient remains on empiric, broad-spectrum antibiotics (vancomycin and piperacillin-tazobactam) and narrows to a targeted regimen when speciation and sensitivities are determined. A peripherally-inserted central catheter (PICC) is inserted in anticipation of a long-term parenteral antibiotic regimen for 6–8 weeks. Our infectious disease colleagues assist in the dosing of antibiotics, mitigating of side effects, and determining of an appropriate length of treatment.
Results
The study population included ten patients with early post-operative infection, consisting of 4 men (40%) and 6 women (60%). The mean age at the time of index surgery was 60.2±19.9 years (range, 22.0–86.0 years). The most common diagnoses included degenerative scoliosis (50%), degenerative kyphosis (30%), idiopathic scoliosis (10%), and tumor (10%). The mean number of levels included in the index fusion was 7.4±4.7. One patient (10%) underwent fusion from the upper thoracic spine to the pelvis, and one patient underwent isolated upper thoracic corpectomy and fusion (10%). Five patients (50%) underwent fusion from the lower thoracic spine (T8-T10) to the pelvis. One patient (10%) underwent fusion from the lumbar spine to the pelvis. Two patients (20%) underwent isolated lumbar decompression and fusion procedures. Five patients (50%) had bone morphogenetic protein insertion at the index procedure. The mean follow-up was 64.4±18.1 months (range, 44.0–98.0 months).
Postoperative infection presented after an average of 41.4±57.5 days (range, 6.0–207.0 days) from the index procedure. Including anesthesia, neuromonitoring, and wake-up time, the average duration of the index procedures was 510.0±169.9 minutes (range, 248.0–747.0 minutes). Clearance of infection was defined as absence of symptoms as outlined by CDC/NHSN criteria including: erythema, warmth, pain or tenderness, and purulent drainage among others (15). Additionally, patient complete blood count, erythrocyte sedimentation rate and C-reactive protein markers were tracked to confirm absence of continue inflammatory response. At final follow-up, none of the ten patients in our series had evidence of continued deep infection. Additionally, no patients required removal of deep hardware. Ten of the ten patients (100%) were able to clear their infection by use of a single stage irrigation and debridement and placement of antibiotic cement (Table 1).
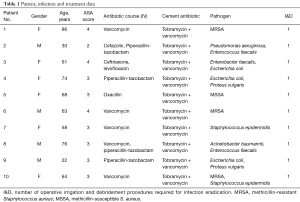
Full table
Discussion
The 2.4% deep infection rate in our series is consistent with the 3–6% reported rate in the literature for instrumented spine surgery (4-7,16). Risk factors for postoperative spinal infection are multifactorial and dependent on both host and surgical factors. The type of procedure, operative duration, patient comorbidities and nutritional status, and various other factors have all been implicated as predictors of postoperative infection (16). Furthermore, surgical arthrodesis and the use of instrumentation are independent factors associated with an increased rate of infection (17). While spinal infections in the absence of implants can be successfully treated with antimicrobial therapy alone, the presence of instrumentation provides an additional treatment dilemma, often requiring aggressive surgical management (9).
It is difficult to interpret previous outcome studies of implant-associated spinal infections given the variability in definitions for deep infection, acuity of infections, treatment protocols, and described outcome measures. Some authors report good outcomes without any loss of function with aggressive early surgical irrigation and debridement (18,19). In a matched cohort analysis, Mok et al. showed that, after successful resolution of deep infection, patients reported a health status similar to matched controls who underwent an uninfected postoperative course (19). However, the extensive treatment period consisting of repeated surgical procedures, prolonged hospitalizations and secondary adverse medical events can be physically and psychologically disabling. Moreover, 25% (4/16) patients in the study by Mok et al. required removal of instrumentation for infection eradication (19). Similarly, in a series of 53 pediatric patients, Ho et al. reported an explantation rate of 31% for postoperative implant-associated infection (20).
In the setting of deep infection, the risk of pseudarthrosis despite retained instrumentation is significantly elevated. Weiss et al. reported a 37.9% pseudarthrosis rate after deep infection and a 64.3% rate in patients with long constructs including the sacrum. The authors supported leaving stable implants in the setting of deep infection, suggesting that biomechanical stability may be more clinically beneficial than complete removal of foreign material from the wound (21). Removal of hardware with the intention of infection clearance is not without complication. Explantation in an unfused, destabilized spine risks neurologic compromise, decreased functional outcomes, loss of surgical correction, and pseudarthrosis (22,23). Furthermore, Collins et al reported that only 46% of patients had stable pain-free spines when managed with routine removal of implants in established fusions (24). Therefore, the goal in the management of implant-associated spine infections should be infection eradication, retention of implantation, and prevention of recurrence while minimizing patient morbidity.
The general orthopaedic surgeon is quite familiar with challenge of treating infections in the setting of implanted hardware. Prosthetic associated infections in joint arthroplasty and traumatology routinely require repeated surgical debridements and removal of foreign bodies for eventual clearance of infection. The failure of treatment and high rate of recurrence with antimicrobial agents has been attributed to biofilm production. Previously thought as an inert barrier to penetration by antimicrobials, biofilms represent a living and rapidly changing bacterial microhabitat. Within biofilms, microorganisms develop into organized communities with advanced cell-to-cell signaling allowing collaborative function similar to multicellular organisms (25). Biofilm resistance to antimicrobial therapy begins with bacterial attachment and increases with biofilm age (26). In one study of S. epidermidis, vancomycin efficacy decreased significantly as the biofilm aged from 6 hours to 2 days (27). The rapidity of antimicrobial resistance highlights the importance of early infection recognition, exploration, and aggressive surgical debridement. The use of dilute bacitracin irrigation solutions (28,29), closed irrigation-suction systems (2), vacuum-assisted wound closure (30), and delayed wound closure (31) have been proposed as potential solutions to the problem of biofilm formation and persistent infection.
For nearly 2 decades, the use of local antibiotic therapy in combination with thorough debridement has been shown to be effective in reducing the incidence of infection in severe open fractures and treatment of local osteomyelitis (32). The efficacy of antibiotic-loaded polymethylmethacrylate (PMMA) cement hinges on its ability to provide high local antibiotic concentrations while minimizing systemic toxicity (33). Two-stage revision arthroplasty with interval placement of a high-dose antibiotic impregnated PMMA spacer is the gold standard treatment for chronic periprosthetic joint infections in North America (12). The use of antibiotic cement in spinal surgery is less widespread. Glassman et al. first reported on the successful use of antibiotic beads to salvage infected instrumented lumbar fusions. In his series, clearance of infection was obtained after a mean of 4.7 (range, 2–10) irrigation and debridement procedures, including an additional procedure for bead removal (29).
In our series, postoperative infection presented after an average of 41.4 days from the index procedure. Consistent with previous reports of increased infection risk with lengthy operative times, our average operative duration was 510 minutes (34,35). After the aforementioned treatment protocol, all patients in the series cleared their infection with a single irrigation and debridement procedure. By retaining the antibiotic cement with a single debridement procedure, the patient avoids the physical and psychological morbidity of a second surgery for cement removal and repeat bone grafting. No patients required removal of implantation for clearance of infection, and there were no signs of persistent infection after an average of 64.6 months of follow-up. results of the patients in this case series represent a significant improvement over the reported outcomes in the literature. In addition to the standard treatment of early aggressive surgical debridement, systemic antibiotics, and local soft tissue management, the success achieved in this study is likely attributed to local antibiotic delivery and a novel cementation technique.
The application of antibiotic-loaded PMMA cement over the exposed instrumentation likely allows for an extremely high antibiotic concentration at the implant interface, where it can be most efficacious. Other studies have emphasized the importance of mechanical and chemical debridement with dilute bactericidal solutions in the disruption of the biofilm layer (28,29). We speculate that the exothermic reaction of cement solidification provides an additional thermal debridement and thus, may increase the permeability of the biofilm to local antibiotic penetration. Moreover, the inert cement structure may resist biofilm adhesion. Minimizing potential neurologic compromise, the placement of cement over exposed hardware prevents the possibility of cement migration into the canal. Additionally, the cement is firmly affixed to the implant surface. Therefore, the need for a subsequent procedure in the operating room for removal of cement, which is typically associated with antibiotic beads, is obviated, and the treatment course becomes significantly more cost effective.
Weaknesses of the study include an outcome of interest that is difficult to define. Successful eradication of infection was defined as a minimum of 18 months free from infection, even though delayed infections are known to occur over 18 months from the index surgery (25). Unfortunately, the greatest limitations of this study are inherent to all case series—the retrospective nature, small number of patients, and lack of a control group. We recognize and respect these limitations; however, many innovative techniques begin with case reports and case series, followed by studies with higher levels of evidence. Chen and Lee (13) previously published a related case report describing the implantation of a permanent antibiotic cement strut for the treatment of cervical pyogenic spondylitis, and this is the first case series in the English literature reporting on permanent implantation of antibiotic cement over exposed instrumentation. Future prospective, randomized controlled trials will provide a more rigorous assessment of our novel protocol and its efficacy.
Conclusions
The sequelae of deep, implant-associated spine infections are devastating. Prolonged hospitalization, long-term intravenous antibiotic therapy, management of secondary complications (36), and the need for repeated debridement procedures in the operating room results in significant morbidity to the patient and economic burden on the entire healthcare system (11). Herein, we described a deep infection treatment protocol involving permanent implantation of antibiotic-loaded PMMA cement. Supplementing aggressive debridement and systemic antimicrobial therapy, this novel technique is effective in preserving spinal instrumentation during infection eradication, preventing infection recurrence, and minimizing operative debridements.
Acknowledgements
The authors acknowledge Christopher LoRusso with Stryker Spine for aiding in photography.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Holloway KL, Henneberg RJ, de Barros Lopez M, et al. Evolution of human tuberculosis: a systematic review and meta-analysis of paleopathological evidence. Homo 2011;62:402-58. [Crossref] [PubMed]
- Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg 1997;86:975-80. [Crossref] [PubMed]
- Davis H. Increasing rates of cervical and lumbar spine surgery in the United States, 1979–1990. Spine 1994;19:1117-23. [Crossref] [PubMed]
- Davne SH, Myers DL. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine 1992;17:S184-9. [Crossref] [PubMed]
- Prothero SR, Parkes JC, Stinchfield FE. Complications after low back fusion in 1000 patients. J Bone Joint Surg [Am] 1966;48:57-65. [Crossref]
- Thalgott JS, Cotler HB, Sasso RC, et al. Postoperative infections in spinal implants. Classification and analysis-A multicenter study. Spine 1991;16:981-4. [Crossref] [PubMed]
- Yuan HA, Garfin SR, Dickman CA, et al. A historical cohort study of pedicle screw fixation in thoracic, lumbar, and sacral spinal fusions. Spine (Phila Pa 1976) 1994;19:2279S-96S. [Crossref] [PubMed]
- Hawkinson N, Schwab F, Kelly B, et al. Major complications after adult spinal deformity surgery: is there a high risk patient profile? Neurosurgery 2009;65:409-10. [Crossref]
- Miksić NG. Spinal infections with and without hardware: the viewpoint of an infectious disease specialist. Eur J Orthop Surg Traumatol 2013;23 Suppl 1:S21-8. [Crossref] [PubMed]
- Gaynes RP, Culver DH, Horan TC, et al. Surgical site infection (SSI) rates in the United States, 1992–1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin Infect Dis 2001;33:S69-77. [Crossref] [PubMed]
- Calderone RR, Garland DE, Capen DA, et al. Cost of medical care for postoperative spinal infections. Orthop Clin North Am 1996;27:171-82. [PubMed]
- Kuzyk PR, Dhotar HS, Sternheim A, et al. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: techniques, controversies, and outcomes. J Am Acad Orthop Surg 2014;22:153-64. [Crossref] [PubMed]
- Chen JF, Lee ST. Antibiotic-polymethylmethacrylate strut: an option for treating cervical pyogenic spondylitis. Case report. J Neurosurg Spine 2006;5:90-5. [Crossref] [PubMed]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. [Crossref] [PubMed]
- Lonstein J, Winter R, Moe J, et al. Wound infection with Harrington instrumentation and spine fusion for scoliosis. Clin Orthop 1973.222-33. [PubMed]
- Veeravagu A, Patil CG, Lad SP, et al. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976) 2009;34:1869-72. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2011;36:556-63. [Crossref] [PubMed]
- Beiner JM, Grauer J, Kwon BK, et al. Postoperative wound infections of the spine. Neurosurg Focus 2003;15. [Crossref] [PubMed]
- Mok JM, Guillaume TJ, Talu U, et al. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine (Phila Pa 1976) 2009;34:578-83. [Crossref] [PubMed]
- Ho C, Skaggs DL, Weiss JM, et al. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine 2007;32:2739-44. [Crossref] [PubMed]
- Weiss LE, Vaccaro AR, Scuderi G, et al. Pseudarthrosis after postoperative wound infection in the lumbar spine. J Spinal Disord 1997;10:482-7. [Crossref] [PubMed]
- Muschik M, Lück W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J 2004;13:645-51. [Crossref] [PubMed]
- Deckey JE, Court C, Bradford DS. Loss of sagittal plane correction after removal of spinal implants. Spine (Phila Pa 1976) 2000;25:2453-60. [Crossref] [PubMed]
- Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17:445-50. [Crossref] [PubMed]
- Patel R. Biofilms and antimicrobial resistance. Clin Orthop Relat Res 2005.41-7. [Crossref] [PubMed]
- Knobloch JK, Von Osten H, Horstkotte MA, et al. Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negative Staphylococcus epidermidis. Med Microbiol Immunol 2002;191:107-14. [Crossref] [PubMed]
- Monzón M, Oteiza C, Leiva J, et al. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn Microbiol Infect Dis 2002;44:319-24. [Crossref] [PubMed]
- Rihn JA, Lee JY, Ward WT. Infection after the surgical treatment of adolescent idiopathic scoliosis: evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine 2008;33:289-94. [Crossref] [PubMed]
- Glassman SD, Dimar JR, Puno RM, et al. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976) 1996;21:2163-9. [Crossref] [PubMed]
- Yuan-Innes MJ, Temple CL, Lacey MS. Vacuum-assisted wound closure: a new approach to spinal wounds with exposed hardware. Spine (Phila Pa 1976) 2001;26:E30-3. [Crossref] [PubMed]
- Stambough JL, Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord 1992;5:277-85. [Crossref] [PubMed]
- Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br 1995;77:93-7. [Crossref] [PubMed]
- Cancienne JM, Tyrrell Burrus M, Weiss DB, et al. Applications of Local Antibiotics in Orthopedic Trauma. Orthop Clin North Am 2015;46:495-510. [Crossref] [PubMed]
- Shen J, Liang J, Yu H, et al. Risk factors for delayed infections after spinal fusion and instrumentation in patients with scoliosis. J Neurosurg Spine 2014;21:648-52. [Crossref] [PubMed]
- Akins PT, Harris J, Alvarez JL, et al. Risk Factors Associated With 30-day Readmissions After Instrumented Spine Surgery in 14,939 Patients: 30-day readmissions after instrumented spine surgery. Spine (Phila Pa 1976) 2015;40:1022-32. [Crossref] [PubMed]
- Massie JB, Heller JG, Abitbol JJ, et al. Postoperative posterior spinal wound infections. Clin Orthop 1992.99-108. [PubMed]


