A psoas splitting approach developed for outpatient lateral interbody fusion versus a standard transpsoas approach
Introduction
Physicians are starting to lean toward the outpatient model rather than the traditional hospital setting with the recent development of technologies and techniques that allow for faster, safer treatments (1,2). Studies have demonstrated the safety of outpatient service looking at outcomes and risk of complication in several specialties (2-7). The Centers for Medicare & Medicaid Services (CMS) has published updates with regards to billing codes, fee structures, and payments to be made to ambulatory surgery centers (ASCs) as it relates to spine surgery (8). This move confirms the growing trend to outpatient surgery.
Minimally invasive lateral lumbar interbody fusion (LLIF) has been adopted as a procedural technique used to achieve fusion decreasing the risks associated with traditional anterior fusion (9). Limitations and complications of the lateral transpsoas approach are motor weakness/palsy and sensory dysesthesia (10-13). These complications are often attributed to muscle trauma resulting from passage through the psoas muscle, direct/indirect nerve injury, hematoma, and/or a combination of causes.
To decrease the risk of complications associated with the lateral transpsoas technique, a psoas splitting approach was developed and performed. This study describes our surgical technique using this approach and discusses the benefits that this method may provide for both the surgeon and the patient.
Methods
The medical records of 84 patients with prospectively collected data between January 2013 and December 2014 were retrospectively reviewed with final 2-year follow-up in December 2016. Group 1 consisted of 44 patients who underwent LLIF via the standard lateral transpsoas approach which was performed prior to the psoas splitting group. Group 2 consisted of 40 patients who underwent LLIF via the psoas splitting approach. IRB approval was obtained for the study as part of a cohort of patients undergoing lumbar fusion. All operations were done by a single surgeon with experience performing procedures in academic, private hospitals and outpatient surgery centers. Patients were only considered for surgery after failed conservative management for at least 6 months. Indications for surgery were lumbar disc herniation, spinal stenosis, and chronic lower back pain with or without radiculopathy. Exclusion criteria for this study included acute severe trauma, fractures, malignancy, infection, unstable chronic medical illnesses, prior lumbar fusions and body mass index (BMI) >42 kg/m2 (2,14,15).
Statistical analysis
Values are expressed as counts or means ± standard error as appropriate. Intergroup comparisons were made using a t-test. Data were analyzed using the SPSS statistical software version 22 (IBM Corp., New York, USA). Power analysis was performed based on complication rate; to obtain a statistical power of 80% and confidence interval of 5%, a total sample size of 60 is required. Tests were considered significant if P<0.05.
Psoas splitting LLIF technique
Positioning
The patient is placed in the left lateral position with the break of the table at the iliac crest. A gel roll/rolled towel is placed two finger breaths under the axilla. Fluoroscopy is positioned across from the surgeon. The C-arm is kept at 90-degree angles and the table is rotated to obtain true AP/lateral projections. An approximate 30-degree break in the table is used. The legs are flexed and foot of bed raised to decrease stretching of psoas muscle. A semi-circumferential taping of the chest is performed, with additional taping starting on the table caudal to the iliac crest across the hip to the contralateral side of table. Taping should be adequate to ensure that the patient does not move during the operation. Fluoroscopy should be rechecked to make sure the patient has not rotated during positioning. The patient is prepped and draped in the normal sterile fashion and the Sagittal Lumbar Interbody Fusion Technology (SLIFT) retractor (SpineFrontier Inc. Malden, MA, USA) clamp is placed on the table opposite the surgeon.
The representative or other operating room personnel should be placed at the foot of the bed for feedback on whether the surgeon is working in an anterior or posterior biased angle.
Incision
Fluoroscopy is used to define the superior and inferior endplates of the operative level. The anterior vertebral body wall and posterior vertebral body are also drawn on the side of the patient. Spatial orientation using information from the patients’ anatomy and integration of information provided by fluoroscopy are imperative to safely performing the operation. The incision is made obliquely following the lines of Langer centered over the operative disc space.
Dissection
Preoperative X-rays and MRI should be checked for the location of the great vessels as well as if there are any posterior loops of colon in the operative field.
Dissection is carried down through the adipose tissue to the fascia of the external oblique. The fascia is incised along the axis of the muscle fibers. A finger is used to spread along the axis of the muscle fibers. The thin fascia layer covering the internal oblique is then encountered and opened using a finger. Dissection is carried along the axis of the internal oblique until the transverse abdominal fascia is encountered and opened using blunt dissection. The retroperitoneal cavity is then entered. The index finger is then hooked and swept north and south along the posterior wall of the abdominal cavity until the transverse processes of the lumbar vertebrae are palpable. The psoas lies just anterior and the fat is swept off the psoas by sweeping the index finger anteriorly over the psoas.
Discectomy/interbody placement
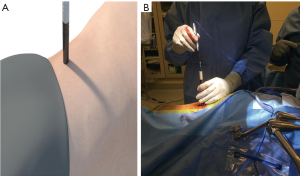
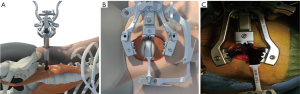
The 8-mm 1st stage dilator is introduced down the posterior side of the finger inside the retroperitoneal cavity, through the psoas and down to the disc space. Anteroposterior (AP) and lateral images are taken to verify the correct operative level and location of the initial dilator. The dilator should be at the junction of the middle and posterior 1/3 of the vertebral body. A blunt K-wire is inserted halfway across the disc space and confirmed by AP and lateral fluoroscopy. The 15-mm dilator with channels for neuromonitoring is introduced. Neuromonitoring is checked in all four quadrants to ensure a safe working distance from the traversing nerve roots, lumbar plexus or any other neural components (Figure 1). Once the action potential of 2–5 mAmp is obtained, an approximate safe zone radius of 7–10 cm which allows an area for adjustments of the SLIFT retractor. The measurement on the dilator is noted as references for the blade lengths and the SLIFT retractor is placed over the dilators (Figure 2A) and confirmed by fluoroscopy. The posterior blade should be the length measured using the dilator with the superior and inferior blades 30–40 mm shorter than the posterior blade. This allows the retractor to open cranially and caudally above the psoas muscle. The posterior blade is positioned to protect the neural structures such as the body posteriorly and traversing nerve roots. The psoas muscle is retracted posteriorly using the posterior blade. This allows direct visualization of any crossing nerve roots. Instead of circumferentially dilating the psoas, which causes increased nerve root tension, a longitudinal split technique is utilized which decreases nerve root tension and retraction. After locking the retractor in place the fourth blade is placed posterior to the anterior longitudinal ligament (ALL) to get adequate visualization of disc space while preserving psoas muscle (Figure 2B,C). The fourth blade can be adjusted or used as a separate retractor to increase direct visualization.
A rectangular annulotomy is performed and any easily removed disc is grabbed with a pituitary rongeur. A dissecting 18-mm Cobb is then advanced along the inferior and superior endplates in a controlled manner. The palm of the hand or light taps from a mallet is/are used to advance the Cobb across the disc space under fluoroscopic visualization. It is important to use an operating room team member at the foot of the operating table to keep the surgeon oriented in an anterior to posterior manner. When the far annulus is encountered it will provide a spring-like or trampoline sensation. The Cobb should be grasped approximately 3–4 mm above the retractor to provide a stop after the annulus is crossed. The Cobb should then be twisted to loosen the contralateral annulus. Disc material is then removed using the pituitary. Endplate prep/disc removal is performed using a ring curette and paddle dilators. A rasping trial, 2 mm smaller than the anticipated final size, is used to complete the endplate preparation. Smooth trials are advanced across the disc space under fluoroscopy. Cage height should be selected by looking at an intact disc cranial or caudal to the operative level. The cage should extend 3–4 mm past the far annulus/lateral vertebral body. If the cage is not driven across completely, the contralateral annulus functions as a hinge and can cause retropulsion of the cage. The cage is packed with demineralized bone matrix (DBM) pure and then inserted using a mallet and confirmed on fluoroscopy (Figure 3A,B). Once the appropriate size cage is advanced across, the bone funnel and ram rod are used to pack the remaining space in the cage with DBM pure (Figure 3C).
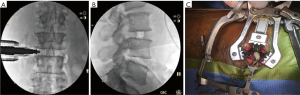
Closure
The SLIFT retractor is slowly removed and the operative field is inspected for any signs of bleeding. Any bleeding must be meticulously controlled. The inferior fascia is closed assuming that the tissue is acceptable. If the tissue is friable, a running closure of the external oblique fascia is performed.
Results
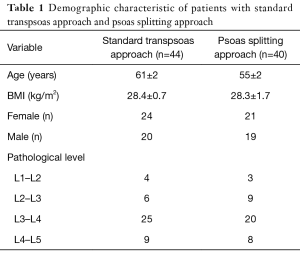
Demographics (Table 1)
Full table
A total of 84 patients were evaluated; we then separated them into two groups. Group 1 comprised of 44 patients with standard approach and group 2 consisted of 40 patients with psoas splitting approach. Females represented 54% of patients overall, however, there was no difference in gender between groups, P=0.850. Overall age and BMI was 58 years and 28.4 kg/m2 respectively. Mean age of group 1 was 61±2 years and group 2 was 55±2 years (P=0.070). Mean BMI for groups 1 and 2 were 28.4±0.7 and 28.3±1.7 kg/m2 respectively, P=0.950.
Functional outcomes
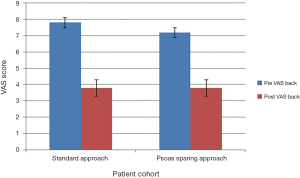
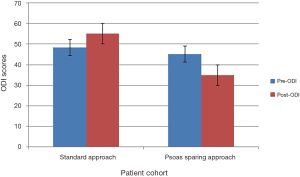
Preoperative functional outcomes for visual analogue scale (VAS) and Oswestry disability index (ODI) scores of group 1 compared to group 2 showed no statistical difference, P=0.527 and P=0.460.
Group 1 mean preoperative VAS back pain scores improved from 7.8±0.3 to 3.8±0.6 at final follow-up, P=0.001. However, mean preoperative ODI score increased from 48.4±3.0 to 55.2±4.0 at final follow-up, P=0.185. In group 2, the preoperative VAS score improved from 7.2±0.4 to 2.7±0.5, P=0.001. Preoperative ODI means reduced from 45.1±5.0 to 34.9±6.0, P=0.239. Outcome scores summarized in Figures 4,5. Statistical comparison of final follow-up outcomes between group 1 and 2 showed statistical difference in VAS scores (P=0.048) and significant improvement in ODI scores (P=0.010).
Analysis of group 1 and group 2 surgical times revealed a statistically significant decrease in the psoas splitting group with operative times of 186±40 and 77±42 min, respectively (P=0.007). This was also true for estimated blood loss, group 1 resulting with 142±35 mL lost and group 2 with 59±12 mL (P=0.041).
Complications
Complications were defined as a new symptom postoperatively (16). In the standard transpsoas approach 65% of patients complained of transient sensory neuropathy corresponding to operative level as compared to 12% of patients who underwent the psoas splitting approach. Using the total neuropathy score, major complications were defined as moderate and severe sensory and motor complications noted to be grade 2 or higher (17) with a higher complication rate in the standard approach (Table 2). Most common complication was sensory dermatome numbness in both groups (11.4% and 5.0%) respectively. Of note, three patients had weakness (grade 2) in standard approach and one patient had inability to walk (grade 3) which resolved in 6 months (17).
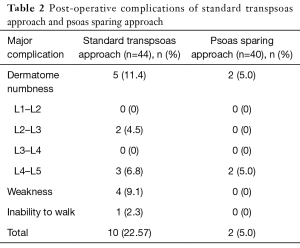
Full table
Discussion
This study aimed to directly compare the relative safety and procedural outcomes of the standard transpsoas and psoas splitting approaches of LLIF. Overall, a statistically significant improvement in VAS and ODI scores was observed for those in patients with psoas splitting approach. Both surgical time and estimated blood loss were statistically lower for the psoas splitting group. In addition, the overall number of complications was higher for patients who underwent the standard lateral transpsoas approach versus the psoas splitting approach.
There is extensive documentation in the literature of complications related to extreme lateral interbody fusion (XLIF), lateral transpsoas interbody fusion (LTIF) and LLIF. Mundis et al. have demonstrated common neurological complications such as weakness/palsy and sensory dysesthesia from 12–75% in their literature review (13). Postoperative thigh symptom frequency has been documented in various clinical experiences ranging from 0–75% (18,19). Despite data suggesting that most post-operative thigh symptoms are ephemeral (20), Sharma et al. found in a preliminary report that 25% of patients with XLIF had transient postoperative anterior thigh pain; another 25% had postoperative hip flexor or quadriceps weakness. Notably, two patients still had the latter deficit 1 year after surgery (21,22). Based upon surgical experience and the data presented in numerous studies (9,23-27), the authors of this paper propose that the above listed complications can be attributed to trauma of the psoas and its respective nerve complex (20). Complications can be attributed to lack of direct visualization and the poor specificity of electromyography monitoring (21). In the study reported by Cummock et al. (19), motor deficits following transpsoas fusion were reported in 24% of patients although no significant changes in electromyography monitoring were identified during the surgical approach. Intraoperative monitoring is unreliable in the upper lumbar roots during traditional transpsoas procedures. There is significant response variation caused by numerous factors including type of neuromonitoring probe as well as the depth and type of anesthesia used (20). The proximity of the lumbar plexus to the lower lumbar area results in complications being significantly more common at L4–5 than other levels (28).
There are several commercially available lateral transpsoas fusion systems with specialized instrumentation which aid in minimally invasive techniques and less exposure surgery (20). With each system there is a learning curve which increases the risk of complications. To minimize risk of damage to neurovascular structures improvements in both localization and intraoperative visualization systems have been introduced. This study demonstrates the use of a technique to guide surgeons in minimizing damage to the psoas muscle, reduces overall postoperative complications.
Strengths and limitations
The authors note the following strengths and limitations.
The main strengths of this study are an adequate sample size and outcomes assessed include patient and surgeon factors which were independently analyzed.
Limitations of this study include the fact that it was a single-surgeon investigation. The surgical experience of the attending surgeon decreases the overall risk of complications over the study period due to the volume of cases performed, number of years in practice in both academic and private setting. This study was also a retrospective review of data collected in two cohort populations prospectively. The increase in ODI scores in group 1 can also be attributed to the fact that the data was retrospective.
Conclusions
The authors have demonstrated good outcomes using a psoas splitting approach to reduce the risk of postoperative neurovascular complications related to the standard lateral transpsoas approach.
Acknowledgements
The authors would like to thank KA Quijada for his help in data collection and analysis.
Footnote
Conflicts of Interest: KR Chin is a shareholder in and receives other benefits from SpineFrontier Inc.; The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of George Washington University (No. 071252).
References
- Best MJ, Buller LT, Eismont FJ. National Trends in Ambulatory Surgery for Intervertebral Disc Disorders and Spinal Stenosis: A 12-Year Analysis of the National Surveys of Ambulatory Surgery. Spine 2015;40:1703-11. [Crossref] [PubMed]
- Chin KR, Pencle FJ, Coombs AV, et al. Lateral Lumbar Interbody Fusion in Ambulatory Surgery Centers: Patient Selection and Outcome Measures Compared With an Inhospital Cohort. Spine 2016;41:686-92. [Crossref] [PubMed]
- Koenig L, Gu Q. Growth of ambulatory surgical centers, surgery volume, and savings to medicare. Am J Gastroenterol 2013;108:10-5. [Crossref] [PubMed]
- Hollenbeck BK, Hollingsworth JM, Dunn RL, et al. Ambulatory surgery center market share and rates of outpatient surgery in the elderly. Surg Innov 2010;17:340-5. [Crossref] [PubMed]
- Pugely AJ, Martin CT, Gao Y, et al. Outpatient surgery reduces short-term complications in lumbar discectomy: an analysis of 4310 patients from the ACS-NSQIP database. Spine (Phila Pa 1976) 2013;38:264-71. [Crossref] [PubMed]
- Billing PS, Crouthamel MR, Oling S, et al. Outpatient laparoscopic sleeve gastrectomy in a free-standing ambulatory surgery center: first 250 cases. Surg Obes Relat Dis 2014;10:101-5. [Crossref] [PubMed]
- Clark N, Schneider DF, Vrabec S, et al. Increased efficiency of endocrine procedures performed in an ambulatory operating room. J Surg Res 2013;184:200-3. [Crossref] [PubMed]
- Association ASC. Medicare's 2015 Final Rule Released; ASCA Efforts Result in Victories for ASCs. Vancouver: October, 2014.
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Anand N, Rosemann R, Khalsa B, et al. Mid-term to long-term clinical and functional outcomes of minimally invasive correction and fusion for adults with scoliosis. Neurosurg Focus 2010;28. [Crossref] [PubMed]
- Houten JK, Alexandre LC, Nasser R, et al. Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine 2011;15:280-4. [Crossref] [PubMed]
- Isaacs RE, Hyde J, Goodrich JA, et al. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine 2010;35:S322-30. [Crossref] [PubMed]
- Mundis GM, Akbarnia BA, Phillips FM. Adult deformity correction through minimally invasive lateral approach techniques. Spine 2010;35:S312-21. [Crossref] [PubMed]
- Chin KR, Coombs AV, Seale JA. Feasibility and patient-reported outcomes after outpatient single-level instrumented posterior lumbar interbody fusion in a surgery center: preliminary results in 16 patients. Spine 2015;40:E36-42. [Crossref] [PubMed]
- Chin KR, Pencle FJR, Coombs AV, et al. Eligibility of Outpatient Spine Surgery Candidates in a Single Private Practice. Clin Spine Surg 2017;30:E1352-8. [Crossref] [PubMed]
- Kardaun JW, White LR, Shaffer WO. Acute complications in patients with surgical treatment of lumbar herniated disc. J Spinal Disord 1990;3:30-8. [Crossref] [PubMed]
- Cavaletti G, Frigeni B, Lanzani F, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst 2007;12:210-5. [Crossref] [PubMed]
- Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine 2010;35:S302-11. [Crossref] [PubMed]
- Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 2011;15:11-8. [Crossref] [PubMed]
- Hardenbrook MA, Miller LE, Block JE. TranS1 VEO system: a novel psoas-sparing device for transpsoas lumbar interbody fusion. Med Devices (Auckl) 2013;6:91-5. [PubMed]
- Kepler CK, Sharma AK, Huang RC. Lateral transpsoas interbody fusion (LTIF) with plate fixation and unilateral pedicle screws: a preliminary report. J Spinal Disord Tech 2011;24:363-7. [Crossref] [PubMed]
- Sharma AK, Kepler CK, Girardi FP, et al. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech 2011;24:242-50. [Crossref] [PubMed]
- Barbagallo GM, Albanese V, Raich AL, et al. Lumbar Lateral Interbody Fusion (LLIF): Comparative Effectiveness and Safety versus PLIF/TLIF and Predictive Factors Affecting LLIF Outcome. Evid Based Spine Care J 2014;5:28-37. [Crossref] [PubMed]
- Dakwar E, Le TV, Baaj AA, et al. Abdominal wall paresis as a complication of minimally invasive lateral transpsoas interbody fusion. Neurosurg Focus 2011;31. [Crossref] [PubMed]
- Lee YS, Park SW, Kim YB. Direct lateral lumbar interbody fusion: clinical and radiological outcomes. J Korean Neurosurg Soc 2014;55:248-54. [Crossref] [PubMed]
- Moller DJ, Slimack NP, Acosta FL, et al. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg Focus 2011;31. [Crossref] [PubMed]
- Talia AJ, Wong ML, Lau HC, et al. Comparison of the different surgical approaches for lumbar interbody fusion. J Clin Neurosci 2015;22:243-51. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine 2011;36:26-32. [Crossref] [PubMed]