
Spinal arachnoid web—a review article
Introduction
Spinal arachnoid web (SAW) is an abnormal thickening of the bands of intradural arachnoid tissue that extend from the pial surface of the dorsal aspect of the spinal cord (1). These webs are sometimes considered as a variant of an arachnoid cyst or remnants of disrupted or collapsed arachnoid cysts or even the incomplete formation of an arachnoid cyst (1-3). SAWs may be formed following a clear history of trauma and this may support the theory of a preceding arachnoid cyst. However, there is the category of non-traumatic arachnoid webs whose etiology is still unknown and consideration is being given to the possibility of it being congenital with an association of a thickened ligamentum flavum (4,5). With Aiyer’s case report included, only 31 cases of SAWs have been reported in the literature of which only 13 have been confirmed at surgery (6,7). These statistics would suggest that SAW is an extremely rare entity and introduces the question as to whether it is indeed so rare or merely being under-diagnosed or under-reported.
Pathophysiology of SAW
The mechanisms culminating in the formation of an arachnoid web remain largely unknown despite SAW being thought to share a common pathophysiology as an arachnoid cyst (1,3). Several theories have been discussed including forceful cerebrospinal fluid (CSF) flow resulting in arachnoid herniation into congenital dural defect, post-traumatic, post-infectious and postoperative etiologies (8-11). Despite these probable culprits, consideration is being given to an idiopathic form of dorsal arachnoid web (DAW) (7). The webs are more commonly located dorsal to the spinal cord and have a predilection for the upper thoracic segment of the spinal cord (1,2,6,7). To date, there is no explanation for this segmental localization of the web (1).
Like webs, the available information relating to the associated syrinx formed is limited. The location of the syrinx varies relative to the associated DAW as it may be caudal but occurs more commonly rostral to the web itself (1-3,12). The syrinx location relative to the arachnoid web is believed to be at the area where the intramedullary pulse pressure is lower relative to the opposite side of the web (13).
Greitz introduced the “Venturi Effect” as probable explanation for the formation of a syrinx stating that the arachnoid web interrupts the transmission of systolic pulse pressure to the distal CSF thereby altering intramedullary pulse pressure. This in effect creates a pressure gradient from the center of the cord outwards resulting in cavitation within the spinal cord (13). This “Suction effect” theory challenged the prevailing theory that increased pulse pressure in the subarachnoid space forces CSF through the spinal cord into the syrinx.
Clinical presentation & investigations of SAW
Patients with DAW present with neuropathic back pain or compressive myelopathic features or radiculopathy including episodic lower extremity weakness, and sensory symptoms and bowel and bladder incontinence. They may be found on clinical examination to have hyperreflexia, spastic paraparesis, clonus and gait instability (6,14). The history may also unveil an antecedent surgery, infection or trauma but this is not always the case. There is an almost 2:1 female:male predominance with age ranging from 4th–7th decade (1).
Investigating these patients may be extensive and a number of preoperative and intraoperative studies have been used to assess these webs. Magnetic resonance imaging (MRI) is the gold standard investigation but has suboptimal sensitivity owing to the relatively thin size of the webs compared to adjacent tissue.
Yamaguchi highlighted that it is difficult for MRI to visualize focal arachnoid lesions and can only suspect webs due to spinal cord deformity and obstructed CSF flow (7). High-resolution sagittal T2-weighted imaging (T2WI) can, however, identify a number of features:
- Extra-medullary transverse band of arachnoid tissue extends to the dorsal surface of the spinal cord;
- Dorsal indentation of the spinal cord.
Together, these comprise the “Scalpel Sign”, which is considered pathognomonic of DAW. It is so named because the mass effect on the dorsal spinal cord from the accumulated CSF is similar to a surgical scalpel with its blade pointing posteriorly (1,6,15). The differential diagnosis of the scalpel sign is beyond the scope of this review, however, those worth mentioning include DAW, dorsal arachnoid cyst (DAC), ventral spinal cord herniation which may be due to occult or repetitive trauma and idiopathic spinal cord herniation (ISCH) which is uncommon (16).
Computed tomography myelogram also can miss webs as it relies on the principle of obstruction to CSF flow and DAWs do not usually cause complete obstruction. Because of its elusive nature to conventional studies, other sequences have been used to improve the sensitivity in diagnosing DAW. MRI with constructive interference in steady state (CISS) has been used to identify webs where myelogram was only suggestive (17).
CINE—cardiac-gated phase-contrast cine-mode MRI in multiple axial planes was able to better identify, correctly localize the SAW and demonstrate a one-way valve like the flow of CSF because of the web (5).
Intraoperative studies are not to be forgotten as they play a vital role as far as SAW management is concerned. At this stage, the extent of web excision now comes into question as overzealous lytic procedure can result in secondary adhesion and relapse of CSF blockage (7). Ultrasound and gentian violet solution have thus far been positively employed to answer this question. As the aims of the operation are to relieve the spinal cord compression and restore normal CSF flow, not only web resection but also further arachnoid lysis may be warranted. This lysis can continue laterally to ensure communication with the ventral arachnoid space. Following laminectomy, ultrasound has to demonstrate the web location prior to durotomy (2). A small volume of diluted gentian violet solution is safe to inject into the subarachnoid space close to the web. Here, hold up or just slowing of contrast confirms CSF flow obstruction but seepage of CSF across the septum is suggestive of alternate CSF pathway such as via the nerve roots (7).
SAW Management decision-making from our experience
Whilst the authors agree with Aiyer and colleagues that surgical resection of the web is curative, the decision has to be first made as to who should be offered surgery (6). In response to Yamaguchi’s statement that surgical resection of the thickened arachnoid membrane is the first choice of treatment; we seek to put forward the argument that the approach to patients with SAW should be individualized (7). This stance is due to the fact that whilst some patients present with clear neurology, others do not and it is in this group of patients where the decision to operate is difficult.
Queen Elizabeth Hospital in Birmingham (QEHB) is a tertiary neurosurgical institute and a regional trauma center that covers the greater Birmingham region with an estimated population of 4.5 million performing in excess of 4,000 emergency and elective neurosurgical procedures each year.
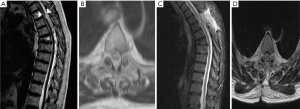
At the QEHB we see approximately ten patients with SAW per annum and our experience with recent patients is that some can be managed conservatively with regular imaging and neurophysiology but some would benefit from surgery. We share 2 of our most recently managed cases with a diagnosis of SAW/spinal arachnoid cyst (SAC) to demonstrate the need for individualized decision making. The first case is a 70-year-old gentleman who developed sudden onset left lower limb weakness (MRC grade 2/5) one week following a fall at which time he suffered an anterior wedge fracture of the sixth thoracic vertebra. MRI imaging showed the scalpel sign (see Figure 1). T3–T4 laminectomy and resection of the possibly cystic arachnoid web formation with lateral fenestration of the thickened arachnoid tissue was performed with neither intraoperative imaging nor dye administration. Postoperatively, his power improved to MRC grade 5/5 except at bilateral L2 myotome, which was 3/5. He had full recovery of sensation prior to discharge and was discharged home ambulating with a Zimmer frame with outpatient physiotherapy. Postoperative electromyogram (EMG) was normal and ten weeks postoperatively he had regained full power in both lower limbs and managed to ambulate unassisted. This clinical presentation with neurologic deficit warrants surgery. In our experience surgery is usually rewarding in symptomatic patients.
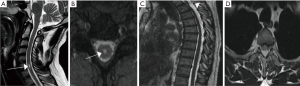
At the other end of the spectrum, we present a 44-year-old man who presented with interscapular pain with no neurological deficit but MRI showed focal anterior cord displacement at T3 level and syrinx evident at C6 (see Figure 2). This patient represents a group of patients with the web but no underlying changes in the cord and minor symptoms where a more conservative approach is recommended. Some patients may present with incidental MRI findings consistent with SAW with or without signal change in the cord. In such cases again a conservative management with clinical monitoring is appropriate. However, symptomatic patients should be offered surgery even without progressive neurological symptoms.
Endoscopic resection has been postulated for the management of spinal intradural arachnoid cysts (18). Minimally invasive intervention almost certainly will reduce the extent of adhesion and thereby lessen the chances of secondary scarring and blockage of CSF flow.
Conclusions
SAW is a rarely reported pathology with varying clinico-radiological presentation and whose etiology remains unknown. In our experience surgical resection/fenestration of the web is usually curative, treatment should however be individualized and take into consideration severity of symptoms, clinical and radiological findings. MRI with CISS imaging is in our view the best imaging modality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reardon MA, Raghavan P, Carpenter-Bailey K, et al. Dorsal thoracic arachnoid web and the "scalpel sign": a distinct clinical-radiologic entity. AJNR Am J Neuroradiol 2013;34:1104-10. [Crossref] [PubMed]
- Sridharan A, Heilman CB. Transverse dorsal arachnoid web and syringomyelia: case report. Neurosurgery 2009;65:E216-7; discussion E217.
- Paramore CG. Dorsal arachnoid web with spinal cord compression: variant of an arachnoid cyst? Report of two cases. J Neurosurg 2000;93:287-90. [PubMed]
- Arai A, Aihara H, Miyake S, et al. Syringomyelia due to thoracic spinal stenosis with ossified ligamentum flavum--case report. Neurol Med Chir (Tokyo) 2011;51:157-9. [Crossref] [PubMed]
- Chang HS, Nagai A, Oya S, et al. Dorsal spinal arachnoid web diagnosed with the quantitative measurement of cerebrospinal fluid flow on magnetic resonance imaging. J Neurosurg Spine 2014;20:227-33. [Crossref] [PubMed]
- Aiyer R, El-Sherif Y, Voutsinas L. Dorsal thoracic arachnoid web presenting as neuropathic pain: 'Scalpel' sign found on MRI. Neuroradiol J 2016;29:393-5. [Crossref] [PubMed]
- Yamaguchi S, Hida K, Takeda M, et al. Visualization of regional cerebrospinal fluid flow with a dye injection technique in focal arachnoid pathologies. J Neurosurg Spine 2015;22:554-7. [Crossref] [PubMed]
- Abou-Fakhr FS, Kanaan SV, Youness FM, et al. Thoracic spinal intradural arachnoid cyst: report of two cases and review of literature. Eur Radiol 2002;12:877-82. [Crossref] [PubMed]
- Mohindra S, Gupta R, Bal A. Intra-dural spinal arachnoid cysts: a short series of 10 patients. Br J Neurosurg 2010;24:679-83. [Crossref] [PubMed]
- Sklar E, Quencer RM, Green BA, et al. Acquired spinal subarachnoid cysts: evaluation with MR, CT myelography, and intraoperative sonography. AJNR Am J Neuroradiol 1989;10:1097-104. [PubMed]
- Srinivasan VM, Fridley JS, Thomas JG, et al. Nuances in Localization and Surgical Treatment of Syringomyelia Associated with Fenestrated and Webbed Intradural Spinal Arachnoid Cyst: A Retrospective Analysis. World Neurosurg 2016;87:176-86. [Crossref] [PubMed]
- Brodbelt AR, Stoodley MA. Syringomyelia and the arachnoid web. Acta Neurochir (Wien) 2003;145:707-11; discussion 711. [Crossref] [PubMed]
- Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev 2006;29:251-63. [Crossref] [PubMed]
- Ruschel LG, Agnoletto GJ, Aurich LA, et al. Dorsal Arachnoid Web and Scalpel Sign: A Diagnostic Imaging Entity. Turk Neurosurg. 2016; discussion 264. [Epub ahead of print]. [Crossref] [PubMed]
- Dua SG, Jhaveri MD. Scalpel sign of dorsal arachnoid web. Neurol India 2016;64:1092-3. [Crossref] [PubMed]
- Haber MD, Nguyen DD, Li S. Differentiation of idiopathic spinal cord herniation from CSF-isointense intraspinal extramedullary lesions displacing the cord. Radiographics 2014;34:313-29. [Crossref] [PubMed]
- Grewal SS, Pirris SM, Vibhute PG, et al. Identification of arachnoid web with a relatively novel magnetic resonance imaging technique. Spine J 2015;15:554-5. [Crossref] [PubMed]
- Endo T, Takahashi T, Jokura H, et al. Surgical treatment of spinal intradural arachnoid cysts using endoscopy. J Neurosurg Spine 2010;12:641-6. [Crossref] [PubMed]


