
Complications associated with intrathecal morphine in spine surgery: a retrospective study
Introduction
Intrathecal morphine (ITM) refers to the single injection of morphine into the subarachnoid space between two lumbar vertebrae, usually L2-L3 or L3-L4 (1). Following injection, morphine exhibits a characteristic gradual spread into the cerebrospinal fluid (CSF) due to its hydrophilic nature (2). By binding to spinal opioid receptors, ITM confers analgesia more rapidly in comparison to alternate modes of administration (intravenous, subcutaneous, oral). The analgesic effect reportedly lasts for 18–24 hours (2). The potential to produce adequate analgesia at low dosages makes ITM an attractive supplement to postoperative pain control regimens.
Barron and Strong first reported the analgesic effect of intrathecal opioids for spine surgery in a 1981 study (3). The opioid-sparing effect of ITM was replicated in subsequent trials and by France et al. in spinal fusion surgery (4-6). Urban et al. similarly demonstrated lower pain scores and morphine consumption in instrumented spinal fusion with a weight-adjusted dose of 20 µg/kg and Ziegeler et al. reported significant reduction in postoperative piritramide consumption with 0.3 mg ITM following posterior lumbar interbody fusion (7,8). Although Pendi et al. reported that adjunctive ITM reduced postoperative pain and analgesic consumption within the first 24 hours following spine surgery, rates of incidental dural tears (IDT), CSF leak, or surgical site infections (SSI) were not analyzed (9). This study aimed to determine the incidence of these complications associated with ITM in a large sample size of posterior instrumented fusions.
Methods
Institutional Review Board approval was obtained prior to initiation of this retrospective study of existing patient records at a tertiary care academic medical center. The Strengthening the Reporting of Observational Studies (STROBE) reporting guidelines for cohort studies were used as a guideline in the reporting of this study (10). A power analysis was performed for sample size estimation based on data from previous studies. Given that SSI are more uncommon than CSF leaks and IDT in spine surgery, rates of SSI were used to determine the sample size necessary to adequately power the study. Previous studies by Collins et al. and Patel et al. reported percentages of SSI in instrumented spinal fusion as 3.7% and 3.8%, respectively (11,12). As a result, the proportion, P of 0.0375 was selected (the midpoint between 3.7% and 3.8%). After selecting α=0.05 and power =0.80, the projected sample size to detect difference in proportions with margin of error ±3% was approximately 308 patients per group.
Identification of the cohort
An ICD-9 code algorithm was used to identify patients that had undergone posterior fusion of the cervical, thoracic, thoracolumbar, lumbar, and sacral regions from January 2010 to January 2016. The exclusion criteria included pediatric patients, surgery for trauma, and surgery for tumor. The ICD-9 code algorithm is provided in the Supplementary (Tables S1,S2).

Full table

Full table
Data abstraction
A list of medical record numbers (MRN), date of service (DOS), and date of birth (DOB) that corresponded to the ICD-9 code algorithm was requested from an Honest Broker. The patient list was then cross-referenced with operative reports to verify posterior instrumented spinal fusion, exclude trauma or tumor, and used to abstract data from the electronic medical record. Non-instrumented spinal fusion cases were excluded. Demographic data such as sex, alcohol use, smoking status, body mass index (BMI), and previous spine operation were recorded. Alcohol use and smoking status were dichotomized such that patients that affirmed use were considered positive and patients that reported former use or quit prior to surgery were considered negative. Body mass indices were recorded as entered in the electronic chart or calculated from the recorded patient weight and height. Co-morbidities included hypertension, diabetes, heart disease, osteoporosis, anxiety, and depression. Intraoperative details included incidence of IDT and injection of ITM. In each patient that received ITM, the surgeon intrathecally injected 0.3 mg of Duramorph (Baxter International, Deerfield, IL, USA) as a 0.4 cc solution into the lumbar spine prior to wound closure. Follow-up, incidence of durotomy, CSF leak, and SSI were each reported as dichotomous outcomes.
Statistical analysis
Patients lost to follow-up were excluded. Patients with missing demographic, co-morbidity, and surgical data were included in the analyses as long as the ITM/control and infection data were available. For sex, use of alcohol, smoking status, co-morbidities, and previous surgery, Pearson chi-squared tests were used to make comparisons. Age and BMI were compared with t-test, two-tailed, and assuming unequal variances. Fisher’s exact test was used to compare the rates of durotomy, CSF leak, and SSI between ITM and control groups. A series of Poisson regressions were conducted to determine the effect of administration of ITM on rates of durotomy, CSF leaks, and SSI separately after adjusting for co-variates and factors that were statistically significant according to t-test or chi-squared test. The level of significance was set at α=0.05. Analyses were carried out using IBM® SPSS® Version 22 (SPSS, Chicago, IL, USA).
Results
Of 733 patients that underwent posterior spinal fusion from January 2010 to January 2016, 175 records were excluded for meeting the one of the following exclusion criterion: pediatric population (n=6), non-posterior instrumented fusion (n=144), trauma (n=13), or tumor (n=12). The remaining 558 records separated into those that included ITM injection (treatment; n=83) and those that did not (control; n=475). Of the 46 patients lost to follow-up, 5 were in the ITM group and 41 were in the control group. The remaining 512 patients were analyzed (78 in the ITM group and 434 in the control group). Record selection is outlined in Figure 1.
ITM group
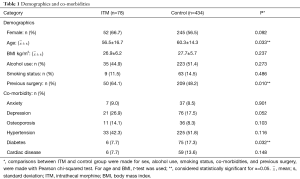
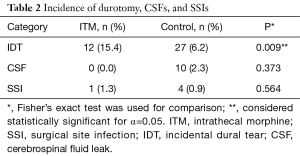
A total of 78 patients were administered ITM in the sample (15.2%). The majority of the ITM group was female (n=52, 66.7%) and aged 18–82 years old (56.5±16.7 years, min: 18, max: 82). Body mass indices of patients in the ITM group varied moderately (26.9±6.2 kg/m2, min: 17.8, max: 47.4). The sample of ITM patients contained several alcohol users (n=35, 44.9%) but few smokers (n=9, 11.5%). The group was characterized by low rates of anxiety (n=7, 9.0%), osteoporosis (n=11, 14.1%), diabetes (n=6, 7.7%), and cardiac disease (n=6, 7.7%). However, incidence of depression (n=21, 26.9%) and hypertension (n=33, 42.3%) were higher. Furthermore, the majority of patients in the ITM group had undergone previous spine surgery (n=50, 64.1%). In the ITM group, IDT (n=12, 15.4%) and SSI (n=1, 1.3%) were each encountered, but CSF leaks were not.
Control group
Of 512 records included in the analyses, the control group comprised 434 patients (84.8%). The group consisted of mostly females (n=245, 56.5%) aged 18–87 years old (60.3±14.3, min: 18, max: 87). Patient body mass indices ranged from 14.7 to 53.5 kg/m2 (27.7±5.7, min: 14.7, max: 53.5). Alcohol use endorsed by the majority of patients in the control group (n=223, 51.4%); alcohol use data was not available for 2 patients. Positive smoking status was recorded for a small number of patients (n=63, 14.5%). Low rates of anxiety (n=37, 8.5%) and osteoporosis (n=36, 8.3%) were reported. By comparison, rates of depression (n=76, 17.5%), hypertension (n=225, 51.8%), diabetes (n=75, 17.3%), and cardiac disease (n=59, 13.6%) were higher. Co-morbidity data were not available for one patient in the control group. In the control group, IDT (n=27, 6.2%), CSF leak (n=10, 2.3%), and SSI (n=4, 0.9%) were encountered in several patients.
Group comparisons
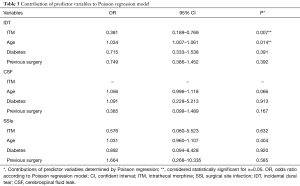
ITM and control groups were compared in terms of demographic, co-morbidity, and surgical variables in Table 1. Patients in the control group were older than those in the ITM group (60.3±14.3 vs. 56.5±16.7, P=0.033). Furthermore, diabetes was significantly more common in the control group (17.3% vs. 7.7%, P=0.032). Also, there were more patients in the ITM group that had a previous spine surgery (64.1% vs. 48.2%, P=0.010). No other demographic, co-morbidity, or surgical variables attained statistical significance. Results of Fisher’s exact can be found in Table 2. Patients that were administered ITM experienced significantly more IDT compared to control (15.4% vs. 6.2%, P=0.009). Differences in occurrence of CSF leaks (0% vs. 2.3%, P=0.373) and SSI (1.3% vs. 0.9%, P=0.564) were each considered statistically insignificant. In order to adjust for demographic, co-morbidity, and surgical variables that attained significance in the crude comparison of the ITM and control groups, Poisson regression was conducted (Table 3). Use of ITM, age, diabetes, and previous spine surgery were included as independent variables. According to the model, ITM was a significant predictor of IDT (OR: 0.381, 95% CI: 0.189 to 0.769, P=0.007). Age also significantly predicted occurrence of IDT (OR: 1.034, 95% CI: 1.007 to 1.061, P=0.014). Neither diabetes nor previous surgery were significant predictors of IDT (P>0.05). ITM administration, age, diabetes, and previous surgery were not significant predictors of CSF leak or SSI (P>0.05) according to Poisson regression.
Full table
Full table
Full table
Discussion
Administration of supplemental ITM remains an attractive option for analgesia following instrumented spinal fusion surgery due to ease of access during lumbar surgery and a opioid-sparing effect at low dosages (8,9). However, the risk of certain complications due to injection of ITM such as IDT, CSF leak, and SSI remain largely unknown (9). We demonstrated that IDT was significantly more common in patients given ITM. Given that intrathecal drug delivery involves puncture of the dura mater, the purpose of this study was to document the rates of IDT, CSF leak, and SSI in order to better define the risk-to-benefit ratio of ITM use and guide surgeons’ clinical decision-making.
The comparison groups differed in terms of the following variables: average age, number of patients with diabetes, and number of patients that had undergone previous spine surgery. These differences are notable because each of the aforementioned variables is a known modifier of risk for IDT, CSF leak, and/or SSI. Advanced age has been described as a risk factor for dural tears, leakage of CSF, and wound infection (13-15). Also, diabetes and previous surgery increase risk of IDT and SSI in spinal operations (14-16). Diabetes has been identified as a significant risk factor for infection following posterior instrumented spinal fusions (17). In order to account for these inter-group differences that may modify the risk of IDT, CSF leak, and SSI, Poisson regression analysis was performed.
IDT is a relatively well-documented complication of spine surgery with an incidence as low as 2.0% to as high as 9.7% in instrumented fusions (18,19). The vast majority of dural tears are identified intraoperatively by the surgical team and can be addressed prior to wound closure (20). According to this study, which found an overall IDT prevalence of 7.6%, dural defects were significantly more common in patients administered ITM compared to control (15.4% vs. 6.2%). This difference remained significant with Poisson regression, which accounted for inter-group differences in age, diabetes prevalence, and past surgical history. Notably, age was also reported to be a predictor for increased occurrence of IDT in this study. Anecdotally, the dura mater has been described as more predisposed to puncture among the elderly (16). Traditional management of IDT involves direct repair with sutures followed by bed rest in the postoperative period. In this study, IDT was repaired directly by suturing the tear and performing the Valsalva maneuver to ensure a water-tight seal prior to wound closure. Notably, IDT neither affects long-term outcomes such as pain or disability nor incidence of further complications such as wound infection (21).
CSF leak may also develop following dural puncture. CSF leaks may be identified intraoperatively or suspected postoperatively due to appearance of symptoms such post dural puncture headaches (PDPH) or persistent clear drainage (14). In this study, the overall prevalence of IDT was low (2.0%) and there were no cases of CSF leak among patients that were administered ITM. Poisson regression revealed no significant predictors of CSF leak. In this review, cases of CSF leak were managed postoperatively with bed rest and observation.
In instrumented spinal fusions, the SSI rate is known to be 3.7–3.8% (11,12). However, SSI rates associated with ITM in instrumented spine surgeries have not been studied in large sample sizes. A total of 5 SSIs were identified in the 512 records that were analyzed (1.0%). Among patients administered ITM and the control group, the difference in occurrence of SSI was considered statistically insignificant. Moreover, after adjusting for the influence of age, diabetes co-morbidity, and previous surgery, injection of ITM was not a significant predictor for infection rates in the Poisson regression model. All SSI were managed with postoperative antibiotics and re-operation for debridement of the wound.
Although this study suggests an increased chance of dural tears due to administration of ITM, these risks may be mitigated by choosing the appropriate needle. O’Connor et al. explored the effect of needle size, type, and dura penetration angle on CSF leakage—finding that former two factors increased CSF leakage but dura penetration angle had no significant effect (22). Previous studies have reported using 25-, 26-, 27-, 29-, and 30-gauge needles to administer intrathecal opioids in adult spine surgery (6-8,23-29). Given that needles with a smaller diameter induce a smaller perforation in the dura mater, these needles require a greater technical skill to ensure optimal drug delivery. As a result, it has been suggested that a balance needs to be struck between the diameter of the needle and ease of effective administration (30). Also, Quincke needles (compared to Whitacre) have been implicated in greater CSF leakage (22,31). Ultimately, choice of needle may constitute one way to reduce the chance of IDT and CSF leakage with use of ITM in spine surgery.
Limitations
The findings of this study are moderated by its limitations. First, although the initial patient list was generated according to a carefully constructed ICD-9 code-based algorithm, a small number of patients that would otherwise be included in the study may have been excluded due to inaccuracies in ICD-9 coding. However, errors in ICD-9 coding were compensated for by cross-checking each patient record with the operative report. Also, despite the large analyzed sample size (n=512), the number of patients in the ITM group was lower than the power analysis estimate. In fact, because ITM injections were administered by only one spine surgeon at the institution, the majority of the patients in the control group were operated on by other surgeons. As a result, there exists potential for differences in surgeon preference and technique between groups to affect risk of SSI, which could not be accounted for in this study. Other limitations stem from the availability of data in the electronic medical record. Co-morbidity data was complete for all but 2 patients. In addition, 7.3% of the records eligible for analysis were excluded due to lack of follow-up; these records may have included cases of IDT, CSF leak, and/or SSI. Certain factors known to modify complication risks, including malnutrition (as measured by pre-operative albumin levels), history of steroid therapy, and ASA score, were not available in the electronic record and therefore could not be accounted for in this study (13,32). However, inter-group differences were compensated for by Poisson regression. Finally, although the findings of this study can be used to direct further exploration into complications associated with ITM administration, the retrospective study design limits the establishment of causal links.
Conclusions
Several studies have documented the effectiveness of adjunctive ITM in reducing postoperative pain following spine surgery. However, the potential complications associated with single-injection ITM in spine surgery have not been thoroughly explored. This retrospective study was performed to document the rates of IDT, CSF leak, and SSI associated with ITM administration in posterior instrumented spinal fusion surgery. Ultimately, patients given ITM experienced significantly more IDT but not CSF leak or SSI. These findings suggest that use of ITM may increase the risk of dural tears. Spine surgeons should be aware of the risk-to-benefit ratio when deciding whether to administer ITM for postoperative pain management.
ICD-9 code algorithm used to identify patient cohort
Acknowledgements
We thank Drs. Tuyen Hoang, PhD and Yanjun Chen, MS for their statistical support.
Funding: This work was partially supported by National Institutes of Health grant UL1 TR001414 from the National Center for Advancing Translational Sciences.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional Review Board approval was obtained at the University of California Irvine (HS#2016-3214). The IRB granted a waiver of consent for this study.
References
- Meylan N, Elia N, Lysakowski C, et al. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth 2009;102:156-67. [Crossref] [PubMed]
- Rathmell JP, Lair TR, Nauman B. The role of intrathecal drugs in the treatment of acute pain. Anesth Analg 2005;101:S30-43. [Crossref] [PubMed]
- Barron DW, Strong JE. Postoperative analgesia in major orthopaedic surgery. Epidural and intrathecal opiates. Anaesthesia 1981;36:937-41. [Crossref] [PubMed]
- O'Neill P, Knickenberg C, Bogahalanda S, et al. Use of intrathecal morphine for postoperative pain relief following lumbar spine surgery. J Neurosurg 1985;63:413-6. [Crossref] [PubMed]
- Blacklock JB, Rea GL, Maxwell RE. Intrathecal morphine during lumbar spine operation for postoperative pain control. Neurosurgery 1986;18:341-4. [Crossref] [PubMed]
- France JC, Jorgenson SS, Lowe TG, et al. The use of intrathecal morphine for analgesia after posterolateral lumbar fusion: a prospective, double-blind, randomized study. Spine (Phila Pa 1976) 1997;22:2272-7. [Crossref] [PubMed]
- Urban MK, Jules-Elysee K, Urquhart B, et al. Reduction in postoperative pain after spinal fusion with instrumentation using intrathecal morphine. Spine (Phila Pa 1976) 2002;27:535-7. [Crossref] [PubMed]
- Ziegeler S, Fritsch E, Bauer C, et al. Therapeutic effect of intrathecal morphine after posterior lumbar interbody fusion surgery: a prospective, double-blind, randomized study. Spine (Phila Pa 1976) 2008;33:2379-86. [Crossref] [PubMed]
- Pendi A, Acosta FL, Tuchman A, et al. Intrathecal Morphine in Spine Surgery: A Meta-analysis of Randomized Controlled Trials. Spine (Phila Pa 1976) 2017;42:E740-7. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. [Crossref] [PubMed]
- Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17:445-50. Erratum in: Eur Spine J 2017. [Crossref] [PubMed]
- Patel H, Khoury H, Girgenti D, et al. Burden of Surgical Site Infections Associated with Select Spine Operations and Involvement of Staphylococcus aureus. Surg Infect (Larchmt) 2017;18:461-73. [Crossref] [PubMed]
- Sin AH, Caldito G, Smith D, et al. Predictive factors for dural tear and cerebrospinal fluid leakage in patients undergoing lumbar surgery. J Neurosurg Spine 2006;5:224-7. [Crossref] [PubMed]
- Blecher R, Anekstein Y, Mirovsky Y. Incidental dural tears during lumbar spine surgery: a retrospective case study of 84 degenerative lumbar spine patients. Asian Spine J 2014;8:639-45. [Crossref] [PubMed]
- Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 2013;4:S392-403. [Crossref] [PubMed]
- Baker GA, Cizik AM, Bransford RJ, et al. Risk factors for unintended durotomy during spine surgery: a multivariate analysis. Spine J 2012;12:121-6. [Crossref] [PubMed]
- Liao JC, Chen WJ, Chen LH, et al. Postoperative wound infection rates after posterior instrumented spinal surgery in diabetic patients. Chang Gung Med J 2006;29:480-5. [PubMed]
- Cammisa FP Jr, Girardi FP, Sangani PK, et al. Incidental durotomy in spine surgery. Spine (Phila Pa 1976) 2000;25:2663-7. [Crossref] [PubMed]
- Khan JA, Yadav SK, Tian R, et al. Outcome of Incidental Unrepaired Dural Tear during Lumbar Spine Surgery: Comparisons of Subfacial Drain with or without Subarachnoid Drain. J Spine Neurosurg 2014;3:7.
- Kalevski SK, Peev NA, Haritonov DG. Incidental Dural Tears in lumbar decompressive surgery: Incidence, causes, treatment, results. Asian J Neurosurg 2010;5:54-9. [PubMed]
- Desai A, Ball PA, Bekelis K, et al. SPORT: Does incidental durotomy affect long-term outcomes in cases of spinal stenosis? Neurosurgery 2011;69:38-44. [Crossref] [PubMed]
- O'Connor G, Gingrich R, Moffat M. The effect of spinal needle design, size, and penetration angle on dural puncture cerebral spinal fluid loss. AANA J 2007;75:111-6. [PubMed]
- Yörükoğlu D, Ateş Y, Temiz H, et al. Comparison of low-dose intrathecal and epidural morphine and bupivacaine infiltration for postoperative pain control after surgery for lumbar disc disease. J Neurosurg Anesthesiol 2005;17:129-33. [Crossref] [PubMed]
- Ross DA, Drasner K, Weinstein PR, et al. Use of intrathecally administered morphine in the treatment of postoperative pain after lumbar spinal surgery: a prospective, double-blind, placebo-controlled study. Neurosurgery 1991;28:700-4. [Crossref] [PubMed]
- Boezaart AP, Eksteen JA, Spuy GV, et al. Intrathecal morphine. Double-blind evaluation of optimal dosage for analgesia after major lumbar spinal surgery. Spine (Phila Pa 1976) 1999;24:1131-7. [Crossref] [PubMed]
- Johnson RG, Miller M, Murphy M. Intraspinal narcotic analgesia. A comparison of two methods of postoperative pain relief. Spine (Phila Pa 1976) 1989;14:363-6. [Crossref] [PubMed]
- Techanivate A, Kiatgungwanglia P, Yingsakmongkol W. Spinal morphine for post-operative analgesia after lumbar laminectomy with fusion. J Med Assoc Thai 2003;86:262-9. [PubMed]
- Yen D, Turner K, Mark D. Is a single low dose of intrathecal morphine a useful adjunct to patient-controlled analgesia for postoperative pain control following lumbar spine surgery? A preliminary report. Pain Res Manag 2015;20:129-32. [Crossref] [PubMed]
- Estañón-García I, López-Jiménez FA. Comparison between intrathecal morphine at high doses vs low doses in spinal lumbo-sacral surgery for postoperative control pain. Revista Mexicana de Anestesiología 2008;31:93-100.
- Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth 2003;91:718-29. [Crossref] [PubMed]
- Westbrook JL, Uncles DR, Sitzman BT, et al. Comparison of the force required for dural puncture with different spinal needles and subsequent leakage of cerebrospinal fluid. Anesth Analg 1994;79:769-72. [Crossref] [PubMed]
- Wimmer C, Gluch H, Franzreb M, et al. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord 1998;11:124-8. [Crossref] [PubMed]



