
Neurologic adverse event avoidance in lateral lumbar interbody fusion: technical considerations using muscle relaxants
Introduction
The most common neurological adverse events (AE) are thigh dysesthetic pain and hip flexor weakness. The retroperitoneal trans-psoas extreme lateral interbody fusion (XLIF) technique has improved over the past decade, with increased efficiency and an emphasis on complication avoidance. After all known procedural safeguards are enacted, the most common failure of neuro-monitoring precision may be the use of non-depolarizing muscle relaxants (MR) for induction, which is the standard of care for anesthesia. Even when non-depolarizing MRs are minimized there is often a small dose given to decrease risk of vocal cord injury with intubation.
The surgical technique for XLIF describes that long-acting MRs should not be used at all, but allows for the use of fast-metabolizing, short-acting MRs to facilitate endotracheal intubation (1). Fast acting MRs such as succinylcholine are preferred over non-depolarizing, long-acting MRs such as Rocuronium because of the adverse effects on electromyogram (EMG). While a positive train-of-four twitch test (TOF) showing adequate muscle function is required prior to performing the surgical procedure (to confirm the absence of paralytic agents), whether or not the use of any muscle-relaxants, even fast-acting ones, impacts procedure-related AE in XLIF remains unknown. The XLIF procedure includes the use of NVM5® (NuVasive, Inc. San Diego, CA, USA), a surgeon-directed neuromonitoring platform that provides real-time discrete-threshold responses in directional orientations during the transpsoas approach and continuous triggered and free-run EMG monitoring throughout the procedure. Due to the requirement for EMG, inhaled gases are limited and nitrous oxide is not used because of the deleterious effect on the twitch test (TOF). Fast acting MR (succinylcholine) is recommended rather than the non-depolarizing, long-acting MRs (e.g., Rocuronium) because of the adverse effect on the EMG. The role of fast-acting MRs on the rate of new AEs in XLIF has not been evaluated, and there are no reports of AEs in XLIF explicitly without the use of any MRs. The hypothesis is performing XLIF without MRs will decrease or eliminate thigh AEs. The purpose of the current study is therefore to present a consecutive series of L3–4 and L4–5 XLIF patients treated by a single surgeon using all procedural safeguards with and without the use of a low dose of non-depolarizing MRs prior to intubation.
Methods
In a retrospective review, 74 consecutive XLIF patients treated at 150 levels without MRs were compared to a group of 125 consecutive XLIF patients treated at 238 levels with non-depolarizing MR. All patients had XLIF at L3–4, L4–5, or both levels. The MR and NMR groups were treated in series, with the MR group preceding the NMR group. The patients in these groups were treated over 3 years, and the primary surgeon had completed more than 350 XLIF procedures prior to this study. The surgeon upon discovering a small dose of Rocuronium was used for intubation, questioned the effect on the neuromonitoring and the NMR group was begun. All procedural technique details were the same for both groups. Perioperative variables were collected, including evoked and free-run EMG readings and postoperative neural and muscular side effects. Hospital records including preoperative patient demographics, anesthesia records describing drugs, dosages, and timing, and progress notes describing postoperative symptoms were studied. These clinical records were reviewed from one, three, and six months for AEs of thigh dysesthetic pain and hip flexor weakness on the ipsilateral side of the XLIF approach.
The standard XLIF technique using NVM5 was performed in all cases, with docking approximately at the 20–30% mark from the posterior border of the disc. Alert-level feedback was attempted in each case to identify the position of the lumbar plexus relative to approach and procedural instrumentation (1). The absence of MRs in the NMR group created small but manageable issues with induction, intubation and maintenance of anesthesia particularly in the chronic pain patient using narcotics before surgery. Anesthetic techniques in all patients included narcotics, minor tranquilizers and intra-venous induction agents. In NMR patients, no MRs was used, with anesthesia modified to use increased doses of the usual agents and an earlier use of inhaled anesthetics. Nitrous oxide was not used during the sequence as nitrous is an inhibitor of neuromonitoring and decreases inhaled oxygen concentration. Intubation was performed following administration of 5 mL of 4% xylocaine to the vocal cords with direct visualization using a McGrath laryngoscopic system (Figure 1). MR patients were treated with unmodified anesthesia including a small dose of Zemuron and succinylcholine before induction.
Differences in baseline demographics between groups were assessed with chi-squared and Fisher’s exact tests for categorical variables and independent samples t-tests for continuous variables. Differences in AEs were assessed with chi-squared and Fisher’s exact tests, with Bonferroni correction for the multiple-test error rate due to the multiple time points. The available sample size provided 80% power to detect a difference of 15% in the rate of AEs between NMR and MR, based on previously reported rates of AEs in XLIF (2). All statistical tests were performed in SAS version 9.4 (Cary, NC, USA) with a two-sided level of significance of α=0.05.
Results
Indications included spondylolisthesis, stenosis, deformity, radiculopathy, pseudarthrosis, degenerative changes and combinations thereof. Five different anesthesiologists were used in the MR group and one anesthesiologist (L.R.) in all NMR patients. All MR patients received non-depolarizing MR Zemuron with dose range (10–50 mg) and some had succinyl choline (50–120 mg) as well (4/125). There were no significant differences comparing doses of MR or different anesthesiologists.
Neuromonitoring data was available for all patients. NMR patients reported a perfect twitch test (>99%) immediately after intubation. MR patients had slower arrival of the twitch and often settled at a lower level (89%±5%, P<0.001). No surgery was attempted until the twitch test was at least 80%. Nerve detection at L4–5 was 98% in the NMR group versus 49% in the MR group (P<0.001) and detection at L3–4 was 47% in the NMR group versus 16% in the MR group (P<0.001).
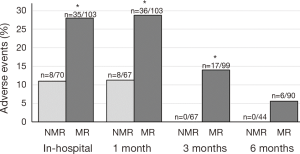
The NMR group had significantly less AE in the hospital (P=0.004), at 1 month (P=0.005), and at 3 months (P<0.001) than the MR group (Figure 2). The uncommon femoral nerve injury with quadriceps weakness was not observed in the NMR group and three MR patients (2.4%) had persistent femoral nerve weakness at 6 months; however, this result was not statistically significant (P=0.296). All NMR AEs resolved by the third month postoperative visit compared with a 17/125 (13.6%, P<0.005) remaining at 3 months and 6/125 persistent AEs at 6 months (4.8%, P=0.178) in the MR group.
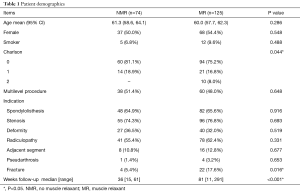
There were no significant differences in age, gender, or smoking history between groups (Table 1). The majority of patients in both groups had a zero Charlson comorbidity index; however, the MR group had more patients with an index of 2 than the NMR group (8.0% vs. 0%, respectively, P=0.032). There were no significant differences in the number of levels performed between the two groups (Table 1). Approximately 50% of cases were single level (Table 1). There were no differences in total AE when comparing single level and two level surgery (P>0.999 for each time point). Furthermore, sub-analyses analyzing single and two level surgery separately produced similar results as the combined analysis, though the statistical power to detect differences was reduced due to the smaller numbers for the sub-analyses.
Full table
The average follow up rate was 96% in the NMR group and 97% in the MR group through 3 months, but dropped to 65% in the NMR group and 86% in the MR group at 6 months. Upon further investigation, patients with AEs at three months had 100% follow up at 6 months, whereas patients without AEs at 3 months had only 77% follow up at 6 months (P=0.025). If all patients who were lost at follow up were assumed to maintain their status as having no AEs from 3 to 6 months, the results of the AEs analysis at 6 months remains unchanged (MR =4.8%, NMR =0.0%, P=0.060).
Discussion
This study specifically compares the XLIF procedure using integrated neuro-monitoring with and without non-depolarizing MRs. This study was born with the realization that Zemuron in small dose was administered to facilitate intubation. The surgeon suggested MRs may affect the neuromonitoring efficiency. While the incidence of AE without MRs in XLIF was significantly lower than that with MRs at early time points, the XLIF without MRs still had an 11% rate of AEs in hospital and at 1 month. These AE completely resolved by the third month without MRs and persisted with MRs at 3 and 6 months. The prevalence of thigh AE in the NMR group, although with early resolution, may be due to the multifactorial nature of the thigh AE. Our rate of AE is substantially lower than published reports of thigh AEs following lateral transpsoas surgery (3). The anesthesia technique with or without the use of MRs has not, to our knowledge, been reported in the literature although an initial twitch test for the presence of MR effect was reported by Tohmeh et al. In a prospective, multicenter study of 102 patients treated with XLIF at L3–4 and/or L4–5, Tohmeh et al. (2) found 18% of patients experienced new postoperative thigh sensory changes and 28% of patients had postoperative hip flexion weakness, both of which were transient, resolving in the early postoperative period. A 3% rate of new postoperative lower extremity weakness was observed with all patients returning to full motor function by 6 months postoperative. The twitch test with the MVN5 was used to assess the presence of MR and a 75% twitch was acceptable. Tohmeh found alert-level (<4.5 mA response thresholds) triggered electromyographic (EMG) feedback in 54% of L4–5 and 32% of L3–4 levels. They did not report the distribution of the AEs from the alert and non-alert level feedback groups; the potential presence of MRs could have been a concern in cases particularly where no alert-level feedback was observed (false negative). In a similar study by Uribe et al. (4), 323 patients were treated with XLIF at L4–5 as part of a prospective, multicenter study. Uribe et al. found a 31% rate of postoperative hip flexion weakness, 13% rate of new postoperative sensory changes and a 4% rate of distal weakness (femoral neuropraxia). In a study of different styles of lateral approach (not all with integrated neuro-monitoring), Cummock et al. (5) reported a 39% rate of thigh pain postoperatively and weakness (hip flexion or motor neural) in 24% of patients. Similarly, Moller et al. (6) reported a 36% rate of hip flexion weakness, 25% rate of thigh sensory changes in patients treated with a variety of lateral trans-psoas approaches. Eighty-four percent of patients with new postoperative deficits had complete resolution by the 6-month time point. In a study by Cheng et al. (7) of neural deficits and complications, patients treated with XLIF were compared to those patients treated with a trans-psoas approach that utilized direct visualization through the psoas muscle without neuro-monitoring. The authors found, overall, a new neurologic deficit (sensory or lower extremity weakness (not hip flexion weakness) rate of 14% in the XLIF and 28% in the direct visualization group. When looking only at single-level procedures, the authors found an expanded difference between the groups with 10% of XLIF and 29% of direct visualization patients experiencing a thigh AE (P=0.03) (7).
In the current study, there were no femoral nerve injuries reported in 75 patients who did not receive MRs and 3/125 who did receive MRs. This may imply an association of the use of non-depolarizing MRs with these rare but severe complications; however, with the number of patients in the current study, we do not have sufficient statistical power to confirm or refute this. Transient hip flexor weakness is the most common AE reported in the literature after a XLIF lateral trans-psoas approach. Investigators have attributed this to the muscle splitting that is required by the approach. There may be no associated sensory deficit but the muscle weakness usually resolves at 3 months (8). The iliohypogastric, ilioinguinal, genitofemoral, and subcostal nerves have overlapping sensory dermatomes and motor innervations. Injury to one or a combination of these nerves during the trans-psoas approach can result in pain, sensory deficits, and abdominal wall weakness (8). The sensory loss of the thigh after L3–4 and L4–5 lateral interbody fusions has been linked to effects on the genitofemoral nerve by several authors (9-11) Findings by Smith et al. (12) suggested that the genitofemoral nerve may be at more risk at the L3–4 level and is consistent with anatomical findings (9-11).
The significant difference in AE’s observed between the two groups and the persistent AE’s and femoral neuropathy in the MR group may suggest increased accuracy of the EMG in the NMR group. In this series our AE result is most likely due to gaining alert-level feedback in each case by the identification and subsequent avoidance of the neural plexus. There are also other factors that may contribute to new postoperative thigh AEs. The presence of baseline comorbidities has been shown to impact medical complications or outcomes in XLIF (13,14) but the relationship between comorbidities and thigh AEs has not been directly studied. We were unable to examine the relationship between individual comorbidities and rates of AEs in the current study. The presence of sub-psoas or intra-psoas hematoma may also present as psoas (hip flexion) weakness (15) but were not specifically evaluated in this study.
The presence of any MRs has the potential to decrease the responsiveness of EMG neuromonitoring in XLIF, which is why the surgical technique and training for XLIF advocate elimination of the use of MRs entirely or to use only fast-metabolizing agents for induction (1). Ozgur found decreased rates of thigh AEs (sensory changes and hip flexion weakness) postoperatively in these XLIF patients when treated entirely without MRs compared to those patients treated with fast-metabolizing MRs during induction followed by a positive twitch test TOF to indicate return of function of the neural motor pathways. Results in the literature are confounded by using different styles of trans-psoas approaches and different techniques for neuromonitoring (3). Authors do not typically describe the anesthesia protocol and there is no way to determine the exact anesthesia protocol used at each institution. The twitch test is the industry standard for the use of MRs in XLIF surgery, and anything less than a perfect twitch may imply a residual MR effect. This makes it difficult to evaluate any potential causal relationship between anesthesia and new postoperative thigh AEs in previous literature.
The no MR anesthesia changes in this study included the use of inhaled anesthetic 2.5–3.5 times minimal alveolar concentration (MAC) during induction. The intubation was done with direct visualization of the vocal cords. Xylocaine delivered into the larynx and trachea makes the intubation less traumatic (Figure 1). During induction and maintenance of anesthesia Diprivan (Propofol) may help in the patient with a history of chronic narcotic use. If the patient does have a muscular response and moves position during the approach and/surgical retraction, additional inhaled gas and Propofol can be helpful without using MR. Twice in the NMR series the surgeon had to start again with dilation and placement of the retractor in the case of excessive movement displacing the retractor on the disc space or altering the position of the patient on the table. The authors have not perceived an increase in anesthesia time since starting the NMR cohort protocol. In the opinion of the anesthesiologist in these cases, discontinuing all MRs as a change to anesthetic technique was not prohibitive and did not change the course of the operation.
While having a single surgeon makes the consecutive series of patient more prone to bias with evolving techniques, the patients in these groups were treated over a relatively short period of time (3 years) and this may make the consistency of technique greater than a multiple surgeon, multicenter study. The surgeon had completed more than 350 XLIF procedures prior to this study. Although the MR cohort was slightly more comorbid than the NMR cohort; excluding these more comorbid patients did not change the results. Future multicenter studies will confirm the generalizability of these results to general practice. Finally while not a limitation, the significant drop off in follow up of the NMR patients after 3 months were, all patients (in both groups) who had no AEs at 3 months. If these patients were assumed to maintain their status as having no AEs at 6 months, the result of the AEs analysis was unchanged.
Conclusions
In this series, the rate of AE in patients treated without any MRs was over 2.5 times lower than in patients treated with MRs, demonstrating a strong association between non-depolarizing MRs used in anesthetic induction of the XLIF patient and the rate of thigh AEs. All resolved by the third postoperative month in the NMR group, whereas some patients in the MR group had persistent deficits at 6 months. No distal weakness was observed in the group without MR. We were unable to examine other factors, such as comorbidities, genitofemoral sensory nerve injury, or multiple punctures of the psoas related to AEs in the present study. Eliminating MRs altogether in XLIF may limit AEs. The development of AEs, however, is multifactorial and the elimination of MRs does not obviate the risk.
Acknowledgements
None.
Footnote
Conflicts of Interest: I Cheng consults for Nuvasive, Cytonics, Spinal Innovation, SpinalCyte, Globus, Stryker, SpineCraft. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was entirely retrospective and did not require an institutional review board approval.
References
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine 2011;14:31-7. [Crossref] [PubMed]
- Lehmen JA, Gerber EJ. MIS lateral spine surgery: a systematic literature review of complications, outcomes, and economics. Eur Spine J 2015;24 Suppl 3:287-313. [Crossref] [PubMed]
- Uribe JS, Isaacs RE, Youssef JA, et al. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur Spine J 2015;24 Suppl 3:378-85. [Crossref] [PubMed]
- Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 2011;15:11-8. [Crossref] [PubMed]
- Moller DJ, Slimack NP, Acosta FL Jr, et al. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg Focus 2011;31. [Crossref] [PubMed]
- Cheng I, Briseno MR, Arrigo RT, et al. Outcomes of Two Different Techniques Using the Lateral Approach for Lumbar Interbody Arthrodesis. Global Spine J 2015;5:308-14. [Crossref] [PubMed]
- Ahmadian A, Deukmedjian AR, Abel N, et al. Analysis of lumbar plexopathies and nerve injury after lateral retroperitoneal transpsoas approach: diagnostic standardization. J Neurosurg Spine 2013;18:289-97. [Crossref] [PubMed]
- Uribe JS. Neural anatomy, neuromonitoring and related complications in extreme lateral interbody fusion: video lecture. Eur Spine J 2015;24 Suppl 3:445-6. [Crossref] [PubMed]
- Uribe JS, Arredondo N, Dakwar E, et al. Defining the safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: an anatomical study. J Neurosurg Spine 2010;13:260-6. [Crossref] [PubMed]
- Uribe JS, Vale FL, Dakwar E. Electromyographic monitoring and its anatomical implications in minimally invasive spine surgery. Spine (Phila Pa 1976) 2010;35:S368-S74. [Crossref] [PubMed]
- Smith WD, Serrano S. Neurologic complications in extreme lateral interbody fusion (XLIF): A comparative analysis of levels L2-3, L3-4, and L4-5. Spine J 2012;12:1. [Crossref]
- Rodgers WB, Cox CS, Gerber EJ. Early complications of extreme lateral interbody fusion in the obese. J Spinal Disord Tech 2010;23:393-7. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Rodgers JA. Lumbar fusion in octogenarians: the promise of minimally invasive surgery. Spine (Phila Pa 1976) 2010;35:S355-S60. [Crossref] [PubMed]
- Berjano P, Balsano M, Buric J, et al. Direct lateral access lumbar and thoracolumbar fusion: preliminary results. Eur Spine J 2012;21 Suppl 1:S37-S42. [Crossref] [PubMed]



