Utilization of the 3D-printed spine model for freehand pedicle screw placement in complex spinal deformity correction
Introduction
Pedicle screws are the biomechanical foundation for spinal deformity correction in modern spine surgery. Various screw insertion techniques have been described including the freehand, fluoroscopic-guided, navigation-assisted, and robotic-assisted methods (1-5). The freehand technique is the preferred pedicle screw insertion method by the senior author (LGL) given its intuitive nature (eyes-on-the-spine), efficient operation flow, shorter surgical time, less cluttered operating room, less chance for equipment contamination, and decreased radiation exposure for both patients and the surgical team.
However, the freehand technique has a steep learning curve since both the entry point and screw trajectory rely on anatomical landmarks such as the superior facet, transverse process, lamina, pars interarticularis, as well as the ventral lamina (6). In patients with severe spinal deformity or multiple prior spinal surgeries, the bony spinal anatomy can be extremely dysmorphic or severely altered, which makes freehand pedicle screw placement more challenging due the absence of, or altered anatomical landmarks.
In recent years, 3D printing has been an emerging technology that is already applied in various medical fields including orthopedics, maxillofacial surgery, cranial surgery, as well as spine surgery (7). The senior author has been utilizing life-size 3D-printed spine models to help surgical planning and freehand pedicle screw placement in cases of severe deformities or significantly altered spinal anatomy. This study aims to describe the senior surgeon’s experience with utilizing 3D-printed spine models to facilitate freehand pedicle screw placement in complex spinal deformity correction.
Methods
All patients who underwent spinal deformity surgery by the senior author (L.G.L) over a 16-month period (September 2015 – December 2016) at the Spine Hospital of Columbia University Medical Center were identified. For patients with severely altered spine anatomy such as in cases of severe kyphoscoliosis and revision surgeries with significant fusion masses, 3D-printed spine models were made preoperatively to facilitate surgical planning and intraoperative freehand pedicle screw placement. Medical records of these patients were reviewed. Patient demographics, pre-operative diagnoses, index procedure, length of procedure, estimated blood loss, and spinal imaging studies were analyzed and recorded.
O-arm imaging was obtained in all of these patients after pedicle screw placement to confirm screw accuracy. Screws were graded as intrapedicular, <2 mm breach, 2–4 mm breach, and >4 mm breach by an independent observer using Vitrea imaging software (Vital Images, Inc., Minneapolis, MN, USA). In addition, anterior breaches >4 mm were also recorded. Patient records were reviewed for procedure specific data and relevant information was recorded and analyzed.
Pre-operative planning
For each case, the 3D-printed spine model was studied carefully before the procedure. Special attention was directed to confirm the curve apex, dysmorphic features, fusion mass, and position of pedicles at each vertebra with intended screw placement. Appropriate entry point of each pedicle screw was marked with a permanent marker based on specific anatomical features at each level. The spinal model was then placed in a sterile, clear plastic cover to be studied intraoperatively to facilitate identification of spinal levels and pedicle screw entry points as needed.
Surgical technique
The spine was first exposed meticulously including all the levels of instrumentation. Care was taken to preserve the soft tissue and facet joint at the most cranial level to minimize risk of proximal junctional kyphosis. All soft tissues were dissected off the spine with monopolar cautery to expose the spinous processes, laminae, facet joints, and bilateral transverse processes. Curettes were then used following initial exposure to remove any residual soft tissue and periosteum. Subsequently, the inferior articular processes at each level were removed using a half-inch osteotome to facilitate visualization of the superior facet and the removed bone fragments were used as autograft. Posterior column osteotomies (PCOs) were then performed as needed according to pre-operative plan. Meticulous hemostasis was achieved at all times using a combination of hemostatic agents, gelfoam, cottonoids, and bone wax as needed.
The pedicle screws were then inserted in a caudal to cranial direction at each level using the technique previously described using a combination of pedicle screw gearshift, ball-tip probe, taps, and K-wire as needed (2,8). When the spinal anatomy was dysmorphic or altered by prior fusion mass, the 3D-printed spine model, which was placed in a sterile plastic bag at the beginning of each case, was brought into the operative field to be compared with the actual spine. Since the model was one-to-one ratio in size, it could be used to facilitate the identification of entry point and trajectory of the pedicle screw. At the completion of the pedicle screw insertion, the O-arm system was used intraoperatively for assessment of screw accuracy by the senior author. Any screw with significant deviation from the planned position was removed and repositioned.
Statistical analysis
Using SPSS 23.0 (Chicago, IL, USA), screw accuracy was calculated. In addition, Pearson’s chi-squared test was used to compare the screw accuracy between the senior surgeon and fellows, as well as the difference in tendency for medial vs. lateral breaches. Screw accuracy was compared to a historical cohort, in which 3D-printed models were not used to assist pedicle screw insertion. Statistical significance was defined by P<0.05.
Results
Patient characteristics
A total of 23 patients (13 women and 10 men) were identified during the study period. The average age was 35.7 years (range, 16–67 years). All patients had complex spinal deformity with pre-operative diagnoses ranging from severe kyphoscoliosis, revision adult degenerative scoliosis, ankylosing spondylitis, hemi-vertebra, and distal junctional instrumentation failure. There were 8 primary cases and 15 revision cases. Twelve patients had a major curve cobb >90 degrees, 11 patients had kyphosis >80 degrees. The average levels of instrumentation were 15.1; PCOs were performed at 119 levels, and three-column osteotomies (3COs) at 21 levels (2 PSOs and 19 VCRs). The average estimated blood loss was 1,753 mL, and average length of procedure was 569.8 mins.
Historical cohort group
A historical cohort of 20 spinal deformity patients (17 females, 3 males) with a total of 352 pedicle screws was used as the control group. The pedicle screws were placed using the standard freehand technique without utilizing 3D-printed spine models. In this group, there were 339/352 screws (96.3%) in the acceptable position, defined as <2 mm breach for this cohort. There were ten screws (2.8%) with 2 to 4 mm breach, while there were three screws (0.9%) with >4 mm breach. Of note, intra-operative O-arm imaging was not used in the historical cohort group, and screw accuracy was assessed on post-operative computed tomography (CT).
Screw accuracy
A total of 513 freehand pedicle screws were placed from T1 to S1 in 23 patients utilizing the 3D-printed spine models. The senior surgeon (LGL) placed 258 screws (50.3%) and spine fellows placed 255 screws (49.7%). Overall, 494 screws (96.3%) were placed in acceptable positions according to the pre-operative plan. The overall screw accuracy (intrapedicular or <2 mm breach) was 84.2%. Among the 81 screws (15.8%) with >2 mm breach, there were 67 lateral breaches compared to 14 medial breaches. Most of the lateral breaches were expected as a result of intended juxtapedicular screw placement in small Type C and D pedicles (9) and did not require screw repositioning. There were only 11 screws (2.1%) that required repositioning due to significant pedicle breaches: six medial and five lateral; in addition, eight screws (1.6%) had >4 mm anterior breach and were shortened accordingly. The use of intra-operative O-arm imaging resulted in repositioning of 19 screws (3.7%) in total.
Compared to a historical cohort with less complex spinal deformities, the current series using 3D-printed spine models achieved a similar percentage of screws placed in the acceptable position (P=0.99), despite the presence of more severe deformities and higher number of revision cases.
Learning curve
Overall, the senior surgeon had a higher percentage of intrapedicular screw placement compared to the fellows with statistical significance (84.1% vs. 76.1%, P=0.02); there was no statistically significant difference between the senior surgeon and the fellows for screws with <2 mm breach (85.7% vs. 82.7%, P=0.37). The senior surgeon had much lower percentage of medial breaches (>0 mm) compared to the fellows (3.1% vs. 10.2%, P=0.001); there was no statistically significant difference in the rate of lateral breaches (12.8% vs. 13.7%, P=0.75). The senior surgeon had a lower chance for screw repositioning compared to the fellows (1.9% vs. 5.5%, P=0.03).
Complications
There was no complication associated with freehand pedicle screw placement in any patient. Specifically, neuromonitoring data was stable in all patients in this study and no patient had any post-operative neurological deficit. In addition, there was no immediate or delayed vascular complications associated with freehand pedicle screw placement in these patients with complex spinal deformity.
Illustrative cases
Case 1—primary adult idiopathic scoliosis
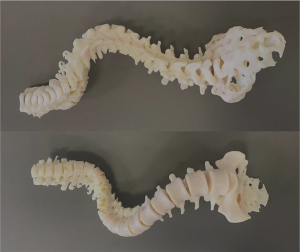
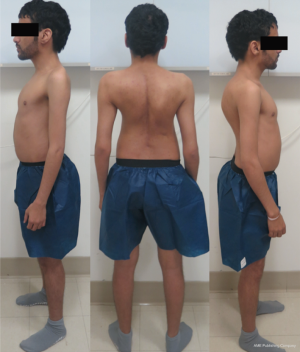
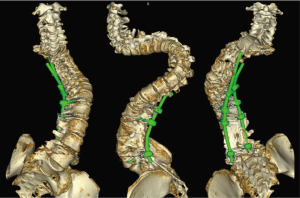
A 27-year-old man with severe adult idiopathic scoliosis presented with worsening deformity and increased chest wall distortion (Figure 1). His neurological examination was unremarkable with full strength, intact sensation and normal reflexes throughout. He stood 5 feet tall and weighed 80 lbs. His thoracic kyphosis measured 125° and right thoracic scoliosis measured 135° with several apical vertebrae touching the chest wall on X-rays (Figure 2). His right rib hump measured >30° on the scoliometer with forward bending. MRI and CT showed no congenital anomalies. CT 3D-reconstruction demonstrated severe kyphoscoliosis (Figure 3). A 3D-printed spine model (Figure 4) was made to facilitate surgical planning and freehand pedicle screw placement.
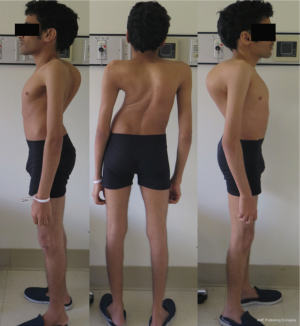
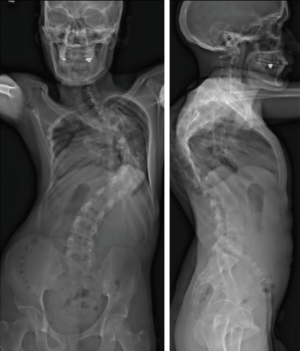
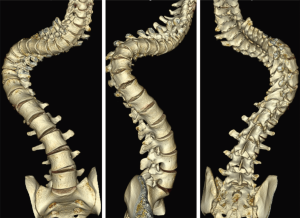
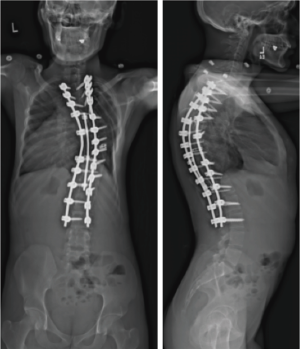
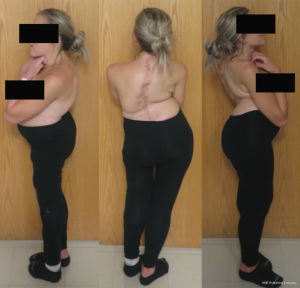
Given his limited baseline pulmonary function (FVC =20%, FEV1 =21%), halo traction was placed for two weeks to optimize his pulmonary function and his nutritional status. At the end of the 2-week Halo traction, his pulmonary function had significant improvement (FVC =36%, FEV1 =34%). He underwent successful posterior spinal fusion from T2-L3 with T9 VCR and T5−12 PCOs without any complications. There was significant improvement in the appearance of the rib hump and the patient remained balanced in both coronal and sagittal plane (Figure 5). Post-operatively, his thoracic curve improved from 135° to 33°, equate to 75.3% curve correction; his thoracic kyphosis improved from 125° to 54.6° (Figure 6). The patient remained clinically well at 18 months post-operatively.
Case 2—revision congenital kyphoscoliosis
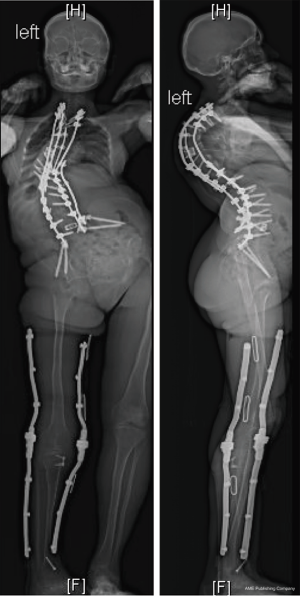
An 18-year-old woman with a complicated past medical history and congenital kyphoscoliosis presented with worsening back pain, progressive shortness of breath, and continued curve progression (Figure 7). She had multiple spinal surgeries previously including spinal cord tumor resection at 2 months of age complicated by left side hemiparesis, growing rods placement and lengthening, definitive spinal instrumentation and fusion complicated by infection and instrumentation failure with subsequent partial instrumentation removal. Her neurological examination revealed chronic paraplegia of her left lower extremity and 4/5 weakness in the left upper extremity. Her motor strength and sensation were grossly intact on the right side with normal reflexes. She stood 4 feet 3 inches and weighed 89 lbs. AP and lateral X-rays (Figure 8) and CT 3D-reconstruction (Figure 9) demonstrated severe kyphoscoliosis with 94° residual thoracic scoliosis and 178° severe angular thoracic kyphosis. A 3D-printed spine model was made to facilitate surgical planning (Figure 10).
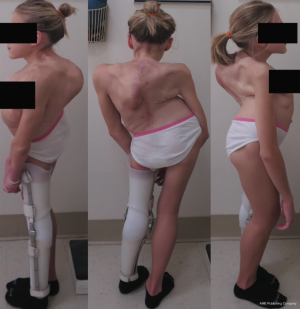
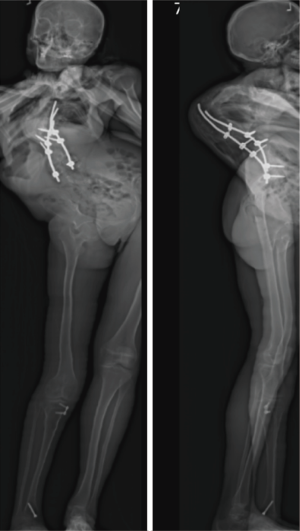

She was placed in Halo traction for 4 weeks to optimize her pulmonary function and nutritional status. She then underwent a two-staged procedure consisting of removal of previous instrumentation and freehand pedicle screw placement from T1 to Pelvis, followed a second stage T8−9 VCRs and T1-pevis fusion. She tolerated the procedure well without any new neurological deficits. There was significant improvement in her spinal deformity with good coronal and sagittal balance (Figure 11). Post-operative X-rays showed improvement of thoracic curve from 94° to 42.5°, and thoracic kyphosis improved from 178° to 91.6° (Figure 12). The patient remained clinically well at the 12-month follow-up visit.
Discussion
In recent years, 3D printing technology has gained tremendous popularity within the medical community with expanding clinical applications (7,10,11). Using this technology, anatomically accurate three-dimensional models can be made from pre-operative imaging to assist in surgical planning. In 2007, Izatt et al. (10) reported their experience using 3D anatomical models in complex spinal surgery. They found that anatomical features were better visualized within 65% of cases compared to traditional 2D imaging such as CT or MRI. Furthermore, they found that 11% of the anatomical details could only be visualized on the 3D model, which influenced the final surgical plan. They also reported a 22% reduction in procedure time for spinal deformity cases. Mobbs et al. (11) recently also reported using 3D printing technology to produce custom spinal implants in addition to surgical planning. To our knowledge, using 3D-printed spine models for freehand pedicle screw placement has not been reported before in the literature.
In the current series, life-size 3D-printed spine models were used to facilitate pedicle screw placement in patients with complex spinal deformities. Preoperatively, the entry point and trajectory of each screw was planned and marked on the spine model. Intraoperatively, the same marked spine model was placed in a clear, sterile plastic cover, and made available for the surgeon as needed. The model was studied intraoperatively to compare with the actual spine to confirm spinal levels, and to determine the appropriate pedicle screw entry point and trajectory based on local anatomy. We found this extremely helpful in patients with severe spinal deformity and significant amount of fusion masses from prior spinal operations. In the current series utilizing the spine models, 494/513 screws (96.3%) were placed in acceptable positions, including screws with <2 mm breach and screws with intended juxtapedicular placement. There were 84.2% of screws with <2 mm breach; among 81 screws (15.8%) with >2 mm breach, there were 67 lateral breaches compared to 14 medial breaches. Among the 67 screws with >2 mm lateral breaches, 62 were intended juxtapedicular screws with good bony purchase in the vertebral body; only 5 of the 67 screws with lateral breaches required repositioning. Most of the lateral breaches were expected as a result of intended juxtapedicular screw placement in small Type C and D pedicles (9) and did not require screw repositioning. Compared to a historical cohort with less complex spinal deformities, the current series using 3D-printed spine models achieved a similar percentage of screws placed in the acceptable position (P=0.99), despite the presence of more severe deformities and higher number of revision cases.
In the current series, there were eleven screws (2.1%) that required repositioning due to significant pedicle breaches: six medial and five lateral. In addition, eight screws (1.6%) had >4 mm anterior breach and were shortened accordingly. The use of intra-operative O-arm imaging resulted in repositioning of 19 screws (3.7%) in total. In contrast, the historical cohort group only had 2/352 screws (0.59%) repositioned, a much lower percentage than in the current series (P=0.02). This discrepancy is most likely explained by the differences in the decision making regarding screw repositioning with and without the use of intraoperative O-arm imaging.
Overall, the senior surgeon had a higher percentage of intrapedicular screw placement compared to the fellows with statistical significance (84.1% vs. 76.1%, P=0.02). This result is not surprising since the freehand technique has a steep learning curve and difference reflects differences in experience. The senior surgeon had much lower percentage of medial breaches (>0 mm) compared to the fellows (3.1% vs. 10.2%, P=0.001). This is likely mostly due to difference in clinical experiences as well. Another additional variable may be that the senior surgeon is almost always positioned on the left side of the patient, which is usually the concavity of the thoracic curve and the vertebrae are rotated away; the right side is more prone for medial breaches in inexperienced hands because the spine is rotated toward the right, and one must direct the screw more lateral at the convexity of the curve. There was no statistically significant difference in the rate of lateral breaches (12.8% vs. 13.7%, P=0.75). The senior surgeon also had a lower rate for screw repositioning compared to the fellows (1.9% vs. 5.5%, P=0.03), which again is largely due to difference in experience.
Several previous studies have reported on the accuracy of pedicle screw insertion using various methods. Macke et al. (4) reported their experience of using robot-assisted thoracic screw placement with an overall accuracy (<2 mm breach) of 92.8% in adolescent idiopathic scoliosis correction. Chiu et al. (1) reported percutaneous pedicle screw placement using fluoroscopy guidance in the lumbar spine and found 97.5% screw accuracy. Avila et al. (12) performed a review of literature and found the mean accuracy for placement of the freehand thoracic screws was 93.3%. Liu et al. (3) also reported no statistically significant difference in accuracy for robot-assisted vs. freehand pedicle screw placement in the lumbosacral region. Our study further demonstrates that freehand screw placement utilizing 3D spine models is safe and effective even in patients complex spinal deformity, with 96.3% screws in acceptable positions according to the pre-operative plan.
Several studies have also reported potential complications related to malpositioned pedicle screws including spinal cord or nerve root injury manifested as neuromonitoring changes or post-operative neurological deficits, dural violation causing CSF leaks, and acute or delayed vascular injuries (13,14). In our series, there was no complication associated with freehand pedicle screw placement in any patient. Specifically, neuromonitoring data was stable in all patients in this study and no patient had any post-operative neurological deficit. In addition, there was no immediate or delayed vascular complications associated with freehand pedicle screw placement in these patients with complex spinal deformity.
Overall, only 2.1% of screws in our study required repositioning due to pedicle violation, including six medial breaches and five lateral breaches. In addition, eight screws (1.6%) had >4 mm anterior breach and all of them were shortened. Intra-operative O-arm imaging was helpful in detecting 19 screws (3.7%) that were malpositioned and potentially reducing the risk of returning to the operating room for revision of the malpositioned screws.
Conclusions
The 3D-printed spinal model is a helpful tool for surgical planning and freehand pedicle screw insertion in patients with complex spinal deformity. It can help spine surgeons to better understand and visualize the complex and altered spinal anatomy in severe spinal deformity and make freehand pedicle screw placement safer.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Lenke is a consultant for and a patent holder with Medtronic. Dr. Lehman is a consultant for Medtronic. The other authors have no conflicts of interest to declare.
Ethical Statement: This study received IRB approval (#AAAR0371). All patients provided informed consent.
References
- Chiu CK, Kwan MK, Chan CY, et al. The accuracy and safety of fluoroscopically guided percutaneous pedicle screws in the lumbosacral junction and the lumbar spine: a review of 880 screws. Bone Joint J 2015;97-B:1111-7. [Crossref] [PubMed]
- Kuklo TR, Lenke LG, O'Brien MF, et al. Accuracy and efficacy of thoracic pedicle screws in curves more than 90 degrees. Spine (Phila Pa 1976) 2005;30:222-6. [Crossref] [PubMed]
- Liu H, Chen W, Wang Z, et al. Comparison of the accuracy between robot-assisted and conventional freehand pedicle screw placement: a systematic review and meta-analysis. Int J Comput Assist Radiol Surg 2016;11:2273-81. [Crossref] [PubMed]
- Macke JJ, Woo R, Varich L. Accuracy of robot-assisted pedicle screw placement for adolescent idiopathic scoliosis in the pediatric population. J Robot Surg 2016;10:145-50. [Crossref] [PubMed]
- Shin BJ, James AR, Njoku IU, et al. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine 2012;17:113-22. [Crossref] [PubMed]
- Lehman RA Jr, Kang DG, Lenke LG, et al. The ventral lamina and superior facet rule: a morphometric analysis for an ideal thoracic pedicle screw starting point. Spine J 2014;14:137-44. [Crossref] [PubMed]
- Tack P, Victor J, Gemmel P, et al. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016;15:115. [Crossref] [PubMed]
- Kim YJ, Lenke LG. Thoracic pedicle screw placement: free-hand technique. Neurol India 2005;53:512-9. [Crossref] [PubMed]
- Sarwahi V, Payares M, Wendolowski S, et al. Pedicle Screw Safety: How Much Anterior Breach Is Safe?: A Cadaveric and CT-Based Study. Spine (Phila Pa 1976) 2017;42:E1305-10. [Crossref] [PubMed]
- Izatt MT, Thorpe PL, Thompson RG, et al. The use of physical biomodelling in complex spinal surgery. Eur Spine J 2007;16:1507-18. [Crossref] [PubMed]
- Mobbs RJ, Coughlan M, Thompson R, et al. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: case report. J Neurosurg Spine 2017;26:513-8. [Crossref] [PubMed]
- Avila MJ, Baaj AA. Freehand Thoracic Pedicle Screw Placement: Review of Existing Strategies and a Step-by-Step Guide Using Uniform Landmarks for All Levels. Cureus 2016;8. [PubMed]
- Feng B, Shen J, Zhang J, et al. How to deal with cerebrospinal fluid leak during pedicle screw fixation in spinal deformities surgery with intraoperative neuromonitoring change. Spine (Phila Pa 1976) 2014;39:E20-5. [Crossref] [PubMed]
- Floccari LV, Larson AN, Crawford CH 3rd, et al. Which Malpositioned Pedicle Screws Should Be Revised? J Pediatr Orthop 2018;38:110-5. [Crossref] [PubMed]