
Limited post-operative dexamethasone use does not affect lumbar fusion: a single institutional experience
Introduction
Dexamethasone is a potent corticosteroid that is routinely utilized as a prophylactic perioperative agent for minimizing pain and reducing opioid use following spine surgeries (1-5). Numerous studies have demonstrated the usefulness of dexamethasone’s anti-inflammatory properties on pain management after surgery (1,2,4). However, its mechanism of action is undesired in spine fusion procedures, as the process of bone fusion is mainly a result of pro-inflammatory responses (6). Furthermore, studies have shown that glucocorticoids favor bone resorption processes over bone healing, which can delay/impair body fusion (6,7).
Currently, perioperative use of exogenous dexamethasone during and after lumbar spine surgery is controversial. The majority of data regarding exogenous dexamethasone use are from animal models. Sawin et al. using an animal model of single level lumbar spinal fusion, demonstrated that animals treated with dexamethasone did not achieve successful spinal fusion. Furthermore, the authors showed decreased tensile strength and stiffness of the fusion mass in the dexamethasone group compared to the control group (8). Although these animal studies demonstrate unsuccessful spinal fusions with dexamethasone use, little is known about the effect of dexamethasone use on lumbar fusion in humans (8,9).
To this end, the aim of this study is to investigate the effect of limited exogenous dexamethasone use on bone fusion after instrumented lumbar arthrodesis.
Methods
Patient selection
This was a retrospective study of 165 patients undergoing elective spinal surgery for treatment of lumbar stenosis and spondylolisthesis. Approval was obtained from the Institutional Review Board prior to study initiation. Hospital records of consecutive adult patients undergoing 1–2 level lumbar decompression and fusion surgeries between January 2013 and December 2014 were reviewed. We included patients 18 years and older with (I) back pain and/or radiculopathy, (II) radiographic evidence of lumbar stenosis, (III) who failed nonsurgical treatment, and (IV) underwent a lumbar decompression and fusion procedure. Patients were excluded if they had severe coexistent pathology that could confound assessment of operative outcome or were unwilling to participate in the study. All patients had a minimum of 1-year of follow-up.
Patients were dichotomized into one of two groups (A and B) based on whether they received dexamethasone during or after lumbar arthrodesis surgery. Group A received dexamethasone and Group B did not receive dexamethasone. The primary outcome of interest was the presence of bony fusion at 12 months after surgery.
Clinical parameters
The evaluated demographic variables included patient age, gender, smoking status and body mass index (BMI). Comorbidities included hypertension, diabetes, coronary artery disease, congestive heart failure, atrial fibrillation, and prior myocardial infarction.
Intraoperative variables included operative time, estimated blood loss (EBL), number of packed red blood cell (PRBC) units transfused, number of fusion levels, incidence of intraoperative spinal cord and nerve root injury and use of subfascial drains. Postoperative complications assessed included the incidence of delirium, urinary tract infection, surgical site infection, ileus, hematoma, myocardial infarction, pulmonary embolism, deep vein thrombosis, motor or a sensory deficit. Length of hospital stay (LOS), and 30-day readmission rate. Fusion rate at 12 months post-operatively was also assessed.
All patients enrolled in the study had post-operative upright lumbar X-rays prior to hospital discharge as well as a CT of the lumbar spine to assess bony fusion within at 12-months of index surgery. Bony fusion was assessed using the criteria previously outlined by Resnick et al. (10).
Statistical analysis
Parametric data were expressed as means ± standard deviation and compared using the Student t-test. Nonparametric data were expressed as median (interquartile range) and compared via the Mann-Whitney U test. Nominal data were compared with the χ2 test. All tests were 2-sided and were statistically significant if the P value was less than 0.05. Statistical analysis was performed using JMP, version 13 (SAS Institute Inc., Cary, North Carolina, USA).
Results
A total of 165 adult patients (dexamethasone cohort: n=58, no dexamethasone cohort: n=107) were included in this retrospective study. No significant difference in patient age between both cohorts was observed (dexamethasone cohort: 58.12±16.25 years vs. no dexamethasone cohort: 61.00±12.95 years, P=0.24) (Table 1). The proportion of males was similar between both groups (dexamethasone cohort: 41.37% male vs. no dexamethasone cohort: 49.53% male, P=0.31) (Table 1). There was no significant difference in BMI between the groups (dexamethasone cohort: 27.67±5.28 kg/m2vs. no dexamethasone cohort: 29.40±7.31 kg/m2, P=0.08) (Table 1). There were no other significant differences in the prevalence of co-morbidities, such as smoking status, CKD, HLD, CAD, A-fib, anemia, and affective disorders between both groups (Table 1).

Full table
The duration of surgery was similar between both groups (P=0.65) (Table 2). Other intraoperative variables, including use of subfascial drains (dexamethasone cohort: 22.41% vs. no dexamethasone cohort: 33.64%, P=0.12) or the occurrence of an incidental durotomy (dexamethasone cohort: 0.00% vs. no dexamethasone cohort: 1.89%, P=0.86), were not significantly different between the groups (Table 2). No patients’ had a nerve root or spinal cord injury. The average dose of exogenous dexamethasone administered to the dexamethasone cohort was 4.65±1.69 mg.
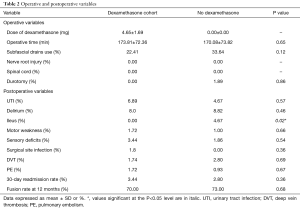
Full table
The prevalence of post-operative complications was similar between both groups, with the exception of ileus (P=0.02) (Table 2). There were no significant differences in the incidence of urinary tract infections (dexamethasone cohort: 6.89% vs. no dexamethasone cohort: 4.67%, P=0.57), delirium (dexamethasone cohort: 8.00% vs. no dexamethasone cohort: 8.82%, P=0.46), motor weakness (dexamethasone cohort: 1.72% vs. No Dexamethasone cohort: 1.00%, P=0.66), sensory deficits (dexamethasone cohort: 3.44% vs. no dexamethasone cohort: 1.86%, P=0.54), surgical site infections (dexamethasone cohort: 1.80% vs. no dexamethasone cohort: 0.00%, P=0.36), DVT (dexamethasone cohort: 1.74% vs. no dexamethasone cohort: 2.80%, P=0.69), PE (dexamethasone cohort: 1.72% vs. no dexamethasone cohort: 0.93%, P=0.67) (Table 2). The 30-day unplanned readmission rate was similar between both groups (dexamethasone cohort: 3.44% vs. no Dexamethasone cohort: 2.80%, P=0.36) (Table 2). At 12 months, 70% of patients in the dexamethasone cohort achieved radiographic evidence of fusion compared with 73% in the no-dexamethasone cohort (P=0.68) (Table 2).
Discussion
In this retrospective cohort study of patients undergoing instrumented lumbar arthrodesis, we demonstrate no significant difference in fusion rate 12 months after index lumbar arthrodesis between patients who received a limited dose of exogenous dexamethasone and patients who did not.
Previous studies regarding the effect of dexamethasone on bone formation and fusion have been limited have been limited to animal models. In an experimental study of twenty-four rabbits who underwent posterolateral lumbar spinal arthrodesis and then were treated with either intramuscular dexamethasone 0.05 mg/kg twice daily, or intramuscular normal saline twice daily, Sawin et al. demonstrated that the rate of fusion after 42 days was significantly lower in the dexamethasone treated group (0% versus 58%) (8). Similarly, in a study of dexamethasone’s effects on bone formation in rats, Ren et al. determined that bone mineral density and markers of bone formation were significantly lower in osteoporotic rats treated with dexamethasone compared to methylprednisone (11). However, Urrutia et al. noted difficulty in applying the results from this study to humans as the dosage of dexamethasone given to the rabbits was much higher than what is given to humans undergoing spinal fusion (9). To better approximate conditions in human patients, these authors performed an experimental study on 36 rabbits in which the animals were given either high dosage of methylprednisolone or saline following posterolateral fusion. After 8 weeks, fusion was achieved in 27.8% of the rabbits given corticosteroid treatment versus 50% in the control group. However, this difference was not statistically significant (9).
While there is a paucity of data on the effect of dexamethasone on fusion rate following lumbar arthrodesis in humans, the effect of dexamethasone on fusion rate following anterior cervical discectomy and fusion (ACDF) in humans has been previously studied. In a prospective randomized controlled trial of 112 patients undergoing multi-level ACDF, Jeyamohan et al. determined that there was a statistically significant difference in the fusion rate at 6 months in patients who received dexamethasone versus those who did not; however no significant difference at 12 or 24 months (5). Similarly, in another prospective randomized study of 50 patients who underwent 1 or 2 level ACDF, Lee et al. found that the intraoperative usage of the corticosteroid triamcinolone also was not linked to a lower long-term rate of fusion. At a mean follow-up time of 21.6 months, all 25 patients randomized to the steroid group achieved bony union versus 24 of the 25 in the control group (12). In a study of 40 patients undergoing combined anterior and posterior cervical fusion and treated with dexamethasone intra-operatively and every 6 hours through post-operative day 1 as a part of the anesthesia protocol, Konya et al. reported that 39 of the 40 patients achieved successful fusion at 12 months (13). Altogether, these results are by and large consistent with our findings, which demonstrate no statistically significant difference in fusion rate 12 months after lumbar arthrodesis.
The osteogenic mechanism of spinal fusion is similar to that of fracture repair and requires the complex interaction of many cell types and signaling pathways, with inflammatory mediators playing a central role in the process (6,14,15). At the initiation of healing, an acute inflammatory phase promotes the recruitment and differentiation of mesenchymal stem cells and the formation of a procallus comprised of granulation tissue (6,15). A repair phase subsequently follows and leads to the formation of a temporary cartilaginous callus that provides an initial weak union at approximately 6 weeks (6). Over time, a remodeling phase occurs in which a balance of osteoclast and osteoblast activity replaces this cartilage with the formation of bone. This turnover and replacement is again regulated by pro-inflammatory signals and usually results in a permanent union at approximately 6 months (6,15). Due to the importance of inflammation throughout the process, the anti-inflammatory properties of steroids have the potential to disrupt healing and lead to nonunion. Although the exact length of the inflammatory phase in spinal fusion is not well known, multiple studies report a higher risk of nonunion with early and immediate postoperative anti-inflammatory drug administration (defined as drug administration intra-operatively to 2 weeks postoperatively) (5,16-18); which may implicate this period as the susceptible inflammatory phase (19).
Currently, data on the effects of dexamethasone on the rate of spinal fusion are limited; however, the effects of other anti-inflammatory medications have been more thoroughly elucidated. In a meta-analysis of the effect of non-steroidal anti-inflammatory drugs (NSAIDs) on spinal fusion, Li et al. found that while the usage of high-dose ketorolac following primary spinal fusion increased the rate of nonunion, usage of normal-dose NSAIDs did not increase the rate of nonunion versus the control (14). Reuben, Ablett, and Kaye also determined in a retrospective study of 434 patients undergoing spinal fusion that high-dose NSAID use resulted in a higher rate of nonunion compared to either low-dose or no-dose NSAIDs (18). Similarly, in a retrospective study of 273 adult patients undergoing 1–2 level posterior lumbar interbody fusions, Lumawig, Yamazaki, and Watanabe demonstrated a dose-dependent inhibitory effect on spinal fusion, most prominent during the immediate postoperative period (17). Together, these studies indicate a possible dose dependent effect of perioperative NSAIDs on spinal fusion. While these results cannot be directly translated to dose-dependent dexamethasone effects, multiple other studies have also shown that corticosteroid-mediated effects on bone are dose dependent (20,21), leading to the possibility that effects of perioperative corticosteroids on spinal fusion may also be dose dependent. However, most of our current understanding of dexamethasone use after lumbar surgery stems from studies in animal models, none of which address dexamethasone-specific dosing thresholds as a risk factor for nonunion (5,12,13) In these studies, the dosage of dexamethasone is much higher than the dose received during human spinal fusion (9), and thus, further research is needed to determine whether a dose threshold exists for dexamethasone usage and for the risk of nonunion.
Overall, the use of dexamethasone during or following spinal surgery remains controversial. Many spine surgeons are reluctant to administer it in the event that it may negatively affect spinal fusion (5). However, due to its anti-inflammatory properties, its remains an important adjunct to reduce pain and opioid use following lumbar arthrodesis (1,2,4). While this study suggests that limited exposure to dexamethasone following lumbar spine surgery may not be associated with lower rates of bony fusion, further studies, including prospective randomized control trials, are needed to better elucidate this relationship.
This study has several limitations that may have implications for its interpretation. The data was obtained from a chart review and is therefore subject to the weaknesses of retrospective analysis; secondly, dexamethasone dosage and administration was not standardized. Dexamethasone was given based on clinical judgement and this could lead to a biased selection. Despite these limitations, this study demonstrates that limited dexamethasone exposure following lumbar spine arthrodesis may not be associated with a decreased fusion rate at 12 months.
Conclusions
Our study suggests that the use of the corticosteroid dexamethasone following instrumented lumbar arthrodesis may not be associated with lower fusion rates 12 months postoperatively. Further studies, including prospective randomized controlled trials, are needed in order to corroborate our results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval was obtained from the RUSH Medical Center IRB (17021801-IRB01-AM04) prior to study initiation.
References
- King JS. Dexamethasone--a helpful adjunct in management after lumbar discectomy. Neurosurgery 1984;14:697-700. [Crossref] [PubMed]
- Watters WC 3rd, Temple AP, Granberry M. The use of dexamethasone in primary lumbar disc surgery. A prospective, randomized, double-blind study. Spine (Phila Pa 1976) 1989;14:440-2. [Crossref] [PubMed]
- De Oliveira GS Jr, Almeida MD, Benzon HT, et al. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2011;115:575-88. [Crossref] [PubMed]
- Nielsen RV, Siegel H, Fomsgaard JS, et al. Preoperative dexamethasone reduces acute but not sustained pain after lumbar disk surgery: a randomized, blinded, placebo-controlled trial. Pain 2015;156:2538-44. [Crossref] [PubMed]
- Jeyamohan SB, Kenning TJ, Petronis KA, et al. Effect of steroid use in anterior cervical discectomy and fusion: a randomized controlled trial. J Neurosurg Spine 2015;23:137-43. [Crossref] [PubMed]
- Pilitsis JG, Lucas DR, Rengachary SS. Bone healing and spinal fusion. Neurosurg Focus 2002;13. [Crossref] [PubMed]
- Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci 2002;966:73-81. [Crossref] [PubMed]
- Sawin PD, Dickman CA, Crawford NR, et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg 2001;94:76-81. [PubMed]
- Urrutia J, Carmona M, Briceno J. Do corticosteroids affect lumbar spinal fusion? A rabbit model using high-dose methylprednisolone. J Orthop Sci 2011;16:439-42. [Crossref] [PubMed]
- Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 17: bone growth stimulators and lumbar fusion. J Neurosurg Spine 2005;2:737-40. [Crossref] [PubMed]
- Ren H, Liang D, Jiang X, et al. Variance of spinal osteoporosis induced by dexamethasone and methylprednisolone and its associated mechanism. Steroids 2015;102:65-75. [Crossref] [PubMed]
- Lee SH, Kim KT, Suk KS, et al. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion: a prospective, randomized study. Spine (Phila Pa 1976) 2011;36:2286-92. [Crossref] [PubMed]
- Konya D, Ozgen S, Gercek A, et al. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci 2009;16:404-9. [Crossref] [PubMed]
- Li Q, Zhang Z, Cai Z. High-dose ketorolac affects adult spinal fusion: a meta-analysis of the effect of perioperative nonsteroidal anti-inflammatory drugs on spinal fusion. Spine (Phila Pa 1976) 2011;36:E461-468. [Crossref] [PubMed]
- Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev 2008;14:179-86. [Crossref] [PubMed]
- Park SY, Moon SH, Park MS, et al. The effects of ketorolac injected via patient controlled analgesia postoperatively on spinal fusion. Yonsei Med J 2005;46:245-51. [Crossref] [PubMed]
- Lumawig JM, Yamazaki A, Watanabe K. Dose-dependent inhibition of diclofenac sodium on posterior lumbar interbody fusion rates. Spine J 2009;9:343-9. [Crossref] [PubMed]
- Reuben SS, Ablett D, Kaye R. High dose nonsteroidal anti-inflammatory drugs compromise spinal fusion. Can J Anaesth 2005;52:506-12. [Crossref] [PubMed]
- Thaller J, Walker M, Kline AJ, et al. The effect of nonsteroidal anti-inflammatory agents on spinal fusion. Orthopedics 2005;28:299-303. [PubMed]
- van Staa TP, Leufkens HG, Abenhaim L, et al. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000;39:1383-9. [Crossref] [PubMed]
- Mitra R. Adverse effects of corticosteroids on bone metabolism: a review. PM R 2011;3:466-71. [Crossref] [PubMed]


