
A less invasive surgery using a full-endoscopic system for L5 nerve root compression caused by lumbar foraminal stenosis
Introduction
L5 nerve root compression occurs at several different intraspinal and extraspinal canal regions. The most frequently affected area is the intracanal region at the L4/5 lumbar disc level. In this region, there are several different pathologies, including lumbar disc herniation (LDH) and lateral recesses stenosis (1). L5 nerve root compression in the extracanal region—the so-called far-out syndrome—also has several different pathologies, including LDH, osteophytes, and entrapment by ligaments (2). Between these intraspinal and extraspinal canal regions, other L5 nerve root compression can be caused by lumbar foraminal stenosis (LFS) (3).
The lumbar foramen is the longest bony canal at the L5/S1 level (4). Therefore, L5 nerve root compression caused by LFS (L5-LFS) occurs more easily than other lumbar nerve root compressions. Moreover, the dorsal root ganglion (DRG) is susceptible to damage by the compression (5); the mean width and length of DRGs gradually increases from L1 to L5 (6). Therefore, L5-LFS is frequently observed as symptomatic LFS in the clinic. Because L5-LFS is associated with orthoarthritis, decreased intervertebral height after degeneration of the lumbar disc, and degeneration of surrounding ligaments (7), its incidence is gradually increasing as the population ages. Clinicians should expect to treat more cases of L5-LFS in the near future.
L5-LFS has been surgically treated with fusion surgery, such as posterior lumbar interbody fusion (PLIF) or transforaminal lumbar interbody fusion (TLIF) (7-9). Recent advancements in minimally invasive lumbar interbody fusion techniques have decreased the invasiveness of these surgical procedures, although they are still invasive compared with a decompressive technique using a full-endoscopic system for percutaneous endoscopic lumbar discectomy (PELD). Based on our experience using full-endoscopic systems, we hypothesized that L5-LFS would be a suitable indication for a percutaneous endoscopic translaminar approach (PETA) (10). In this retrospective analysis, we summarize our experience, identify contraindications for PETA, and present the operative outcomes of PETA for the treatment of L5-LFS.
Methods
Between November 2016 and December 2017, 10 consecutive patients with L5-LFS underwent PETA with a 7-mm diameter spinal full-endoscopic system (Richard Wolf GmbH, Knittlingen, Germany). All patients had radiculopathy that was resistant to medical treatment, epidural steroid injection, and/or nerve block. To clarify the surgical benefits of PETA, we excluded patients with bilateral L5-LFS or L5-LFS with coexisting LDH and/or spondylolysis.
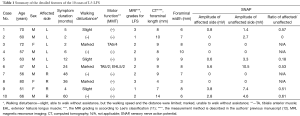
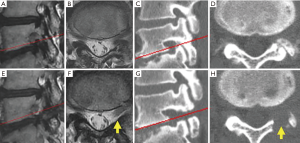
For all patients, PETA was conducted at only one vertebral level by a single surgeon (Hisashi Koga). Neurological examination, electrophysiological examination, preoperative magnetic resonance imaging (MRI), and computed tomography (CT) were used to diagnose L5-LFS. L5-LFS was graded according to Lee’s classification on sagittal T2-weighted MRI (Table 1, Figure 1) (11). The presence of osteophytes protruding to the foramen and decreasing foraminal length/width were evaluated on sagittal CT scan (Table 1, Figure 1C) (12). The laterality of preoperative sensory nerve action potential (SNAP) derived from the peroneal nerve was also used for preoperative evaluation (Table 1) (13).
Full table
Patients were followed for an average of 13.2 months (range, 6–19 months) postoperatively. Preoperative and postoperative neurological statuses were evaluated using the modified Japanese Orthopaedic Association (mJOA) score. The recovery rate was determined as follows: recovery rate = postoperative mJOA − preoperative mJOA/23 (full score) − preoperative mJOA score ×100. Corresponding leg pain was also evaluated using the Numerical Rating Scale (NRS) score.
Surgical technique
Patients were carefully log-rolled into the prone position. The surgery was performed with the patient under general anesthesia and simultaneous motor evoked potential monitoring. During the operation, a fluoroscope was placed across the center of the operating table to ensure appropriate positioning.
An 8-mm skin and fascia incision was made 15–20 mm lateral from the midline at the medial border of the L5 pedicle under fluoroscopic guidance (anteroposterior view), after which an obturator was inserted onto the dorsal surface of the L5 vertebral laminae (Figure 2). Following the insertion of an obturator, a 30°-angled working sheath with a diameter of 7 mm was inserted (Figure 2B). Then, an endoscope (diameter of working channel: 4.1 mm) was inserted. The surface of the vertebral laminae was exposed by forceps and a bipolar radiofrequency electrode system was used (Elliquence, Baldwin, NY, USA). The keyhole entrance is small enough to prevent iatrogenic spondylolysis caused by the destruction of pars interarticularis; however, the area of bone removal is enlarged in the deep part of the hole.
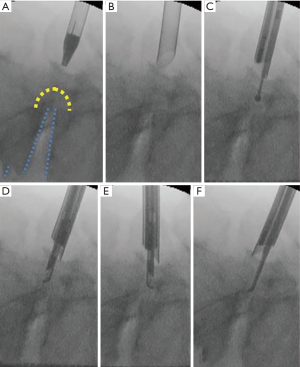
The vertebral laminae were thinned by using a high-speed drill with a diameter of 3.5 mm (NSK-Nakanishi Japan, Tokyo, Japan) (Figure 2C). Subsequently, the residual thin layer was removed with a small Kerrison rongeur (Figure 2D,E). After removal of the dorso-caudal part of the foramen, the lateral part of the yellow ligament and the medial part of the superior articular process (SAP) appear (Figure 3). Further removal of the SAP is required for release of the outlet of the foramen. After completion of the release, the probe can be easily inserted into the extraforaminal region (Figure 2F).

Although the extent of removal of the floating yellow ligament detached from surrounding bone structures was different in each case, we confirmed the decompression by improvements in the intraoperative motor evoked potential monitoring. We also confirmed appropriate bone removal on postoperative CT scan. After decompression, the working sheath was carefully removed and the skin was closed with a single suture.
Results
Ten patients were registered for this study; seven patients were male and three were female. The mean patient age was 62.2 years (range, 47–80 years). Because a definitive determination of LFS is relatively difficult, we diagnosed LFS based on a comprehensive analysis of MRI, CT, and electrophysiological analyses (Table 1). We only performed PETA for unilateral L5-LFS without LDH and/or spondylolysis.
The mean operative time was 77.6 (range, 51–110) min, and the mean blood loss was negligible in all patients (Table 2). We observed no complications in this case series. The mean postoperative hospital stay was 2.6 days (range, 1–5 days); it was particularly for patient 8 because of a lack of improvement in her leg pain (Table 2). The mean mJOA score improved significantly from 8.2±6.01 to 16.3±5.44; the recovery rate of this score was 58.2%±30.1%. The mean NRS scores also improved significantly from 7.4±1.43 to 2.3±2.19 (Table 2).

Full table
During the follow-up period (range, 6–19 months; average, 13.2 months), leg pain stemming from L5-LFS improved in nine cases. A representative case (patient 6) is shown in Figure 2. This 47-year-old man presented with lower back pain and left leg pain (L5 dermatomes) that started 2 years before visiting our outpatient clinic. Neurological examination revealed a negative straight leg raise on both sides but severe muscle weakness (tibialis anterior: 5/2, extensor hallucis longus: 5/2). Lumbar MRI revealed grade 2 LFS, which is moderate LFS with perineural fat obliteration in the four directions without morphologic changes to the nerve root (11), at the L5/S1 disc level (Figure 1A,B). CT scan demonstrated decreasing foraminal length and width due to degeneration of the lumbar disc and hypertrophy of the SAP (Figure 1C,D, Table 1). Immediately after the operation, the patient’s leg pain improved. At 10 months after surgery, the patient’s severe muscle weakness was also improved (tibialis anterior: 5/4, extensor hallucis longus: 5/3), allowing him to jog and participate in martial arts. Postoperative MRI and CT revealed the appearance of fat tissue around the corresponding DRG and enlargement of the foramen, respectively (Figure 1E,F,G,H).
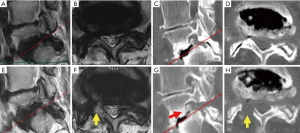
Leg pain persisted postoperatively in only case 8, the oldest patient. This 80-year-old female also had spondylolisthesis (Meyerding grade 2) and degenerative scoliosis (Cobb’s angle: 25) (Figure 4). Although postoperative CT revealed sufficient removal of the dorsal part of the foramen, the appearance of fat tissue around the DRG was not observed on MRI. This patient had a large osteophyte on the ventral side of the lumbar foramen (Figure 4G, red arrow). Such an osteophyte was not observed in any other case.
Discussion
LFS is a pathological condition in which degenerative changes of the vertebral foraminal component cause entrapment of nerve roots (7). Chronic compression of the DRG, which is located at the vertebral foramen and contains pain receptors, is the reason for marked pain; the condition is intractable (15). Fusion surgery, such as PLIF or TLIF, was the previous standard treatment for this entrapment (7-9). Foraminal decompression without lumbar interbody fusion is a potential strategy for a minimally invasive treatment alternative. We therefore analyzed the results of L5-LFS treated by PELD via the PETA and investigated the appropriate operative indications for PETA to prevent persistent pain.
In this article, we summarized 10 cases of L5-LFS treated by PELD via the PETA. The mean recovery rate based on the mJOA score was 58.2%; the mean preoperative and postoperative NRS scores were 7.4 and 2.3, respectively. Although the follow-up period (range, 6–19 months; average, 13.2 months) was not long, the operative outcomes were not inferior compared to previous outcomes of fusion surgery for LFS (16,17). Sclafani et al. analyzed the data for degenerative lumbar diseases (of which 14% were LFS) treated by TLIF and reported that mean preoperative and postoperative visual analog scale (VAS) scores were 5.4 and 1.7 (follow-up period: 1 year) (16). Kim et al. treated LFS by PLIF and reported that mean preoperative and postoperative VAS scores were 8.1 and 3.4 (follow-up period: >2 years) (17). NRS and VAS are similar scores for leg pain.
Recently, several investigators have attempted to treat LFS using minimally invasive strategy. For example, Kim et al. applied transforaminal balloon adhesiolysis to LFS and reported favorable outcomes (18,19). Although successful responses were reported for approximately 20% of enrolled patients with LFS (n=38) (19), indications still need to be carefully defined. Especially for elderly patients, the pathogenesis of LFS includes degenerative changes, such as hypertrophy of the SAP and decreased disc height; thus, at least partial decompression of the foraminal components is required.
With regard to its invasiveness, full-endoscopic spinal surgery is situated between fusion surgery and the previously mentioned catheterized treatment. Because full-endoscopic spinal surgery has the potential to achieve adequate decompression without fusion, several endoscopic spinal surgeons are also trying to treat LFS. Anthony T. Yeung, who is one of the pioneers of full-endoscopic spinal surgery, has been tackling the treatment of LFS from the early stages of development (20). McGrath et al. also treated LFS secondary to fusion surgery and reported satisfactory outcomes (21). Ahn et al. reported excellent operative outcome of LFS treated via transforaminal approach (22). However, even when using a full-endoscopic system for the treatment of L5-LFS, the operative route should be carefully considered. We approach the target foramen from inside to outside (10), but most of the studies is approaching from outside to inside (20-23). Although Ahn et al. emphasized that enough removal of medial part of SAP is the important point of transforaminal approach (22), the decompression can be achieved at the early stage of our procedure. At present, we have no clear answer as to which operative route is a better strategy for L5-LFS. Thus, it is necessary to determine the appropriate operative indications for each approach; otherwise, our approach has no limitations for the high iliac crest or hypertrophied sacral ala (22).
It is also necessary to understand the limitations of our own procedure. Patient 8’s leg pain did not improve, even after appropriate decompression of the dorsal bone structure of the lumbar foramen. The foraminal length and width were extremely narrow in this patient (4 and 3 mm, respectively) compared with the successful cases (Table 1). The reason for this narrowness is the highly degenerative status of the corresponding spine and disc, including decreasing vertebral height, spondylolisthesis (Meyerding grade 2), and large osteophytes located on the ventral side of the foramen. We excluded cases with coexisting LDH and/or spondylolysis in this study; however, it seems that spondylolisthesis (Meyerding grade ≥2) also should have been excluded from this study. Furthermore, a narrow foramen (foraminal length <7 mm, foraminal width <6 mm) and/or large osteophytes on the ventral side of the foramen may be contraindications for our endoscopic technique of L5-LFS. Further improvements to the surgical technique are required to extend the application of this approach. At the present time, fusion surgery should still be considered for patients with the previously mentioned conditions.
Conclusions
The preliminary results obtained during our short follow-up period show that PETA is feasible for the treatment of unilateral L5-LFS. However, a highly degenerative condition such as spondylolisthesis (Meyerding grade ≥2), a narrow foramen, and a large osteophyte located on the ventral side of the lumbar foramen may be contraindications for this procedure.
Acknowledgements
We thank all the operating room staff for their technical assistance and the medical records clerks who helped to collect patient data. We also thank all radiological department staff for recording CT and MRI data. This work was partly supported by a grant from the Iwai Medical Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by ethics committee of the Iwai Medical Foundation, and informed consent was obtained from the patients for publication of this study and any accompanying images.
References
- Hayashi A, Oshima Y, Shiboi R, et al. Microendoscopic Posterior Decompression for the Treatment of Lumbar Lateral Recess Stenosis. J Spine 2016;5:1-5. [Crossref]
- Wiltse LL, Guyer RD, Spencer CW, et al. Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine (Phila Pa 1976) 1984;9:31-41. [Crossref] [PubMed]
- Hasegawa T, Mikawa Y, Watanabe R, et al. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine (Phila Pa 1976) 1996;21:1005-9. [Crossref] [PubMed]
- Silverstein MP, Romrell LJ, Benzel EC, et al. Lumbar dorsal root Ganglia location: an anatomic and MRI assessment. Int J Spine Surg 2015.9. [PubMed]
- van Roy P, Barbaix E, Clarijs JP, et al. Anatomical background of low back pain: variability and degeneration of the lumbar spinal canal and intervertebral disc. Schmerz 2001;15:418-24. [Crossref] [PubMed]
- Silav G, Arslan M, Comert A, et al. Relationship of dorsal root ganglion to intervertebral foramen in lumbar region: an anatomical study and review of literature. J Neurosurg Sci 2016;60:339-44. [PubMed]
- Orita S, Inage K, Eguchi Y, et al. Lumbar foraminal stenosis, the hidden stenosis including at L5/S1. Eur J Orthop Surg Traumatol 2016;26:685-93. [Crossref] [PubMed]
- Watanabe K, Yamazaki A, Morita O, et al. Clinical outcomes of posterior lumbar interbody fusion for lumbar foraminal stenosis: preoperative diagnosis and surgical strategy. J Spinal Disord Tech 2011;24:137-41. [Crossref] [PubMed]
- Fujibayashi S, Neo M, Takemoto M, et al. Paraspinal-approach transforaminal lumbar interbody fusion for the treatment of lumbar foraminal stenosis. J Neurosurg Spine 2010;13:500-8. [Crossref] [PubMed]
- Koga H. Improved percutaneous endoscopic translaminar approach for lumbar foraminal stenosis at L5/S1. Mini-invasive Surgery 2017;1:3-5. [Crossref]
- Lee S, Lee JW, Yeom JS, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol 2010;194:1095-8. [Crossref] [PubMed]
- Yokosuka J, Oshima Y, Kaneko T, et al. Advantages and disadvantages of posterolateral approach for percutaneous endoscopic lumbar discectomy. J Spine Surg 2016;2:158-66. [Crossref] [PubMed]
- Ando M, Tamaki T, Kawakami M, et al. Electrophysiological diagnosis using sensory nerve action potential for the intraforaminal and extraforaminal L5 nerve root entrapment. Eur Spine J 2013;22:833-9. [Crossref] [PubMed]
- Ishibashi K, Oshima Y, Inoue H, et al. PETA against left L5 nerve root compression caused by LFS. Asvide 2018;5:743. Available online: http://www.asvide.com/article/view/27106
- Lin XY, Yang J, Li HM, et al. Dorsal root ganglion compression as an animal model of sciatica and low back pain. Neurosci Bull 2012;28:618-30. [Crossref] [PubMed]
- Sclafani JA, Raiszadeh K, Raiszadeh R, et al. Validation and analysis of a multi-site MIS Prospective Registry through sub-analysis of an MIS TLIF Subgroup. Int J Spine Surg 2014.8. [PubMed]
- Kim EH, Kim HT. En bloc partial laminectomy and posterior lumbar interbody fusion in foraminal spinal stenosis. Asian Spine J 2009;3:66-72. [Crossref] [PubMed]
- Kim SH, Choi WJ, Suh JH, et al. Effects of transforaminal balloon treatment in patients with lumbar foraminal stenosis: a randomized, controlled, double-blind trial. Pain Physician 2013;16:213-24. [PubMed]
- Kim DH, Cho SS, Moon YJ, et al. Factors Associated with Successful Responses to Transforaminal Balloon Adhesiolysis for Chronic Lumbar Foraminal Stenosis: Retrospective Study. Pain Physician 2017;20:E841-8. [PubMed]
- Yeung A, Gore S. Endoscopic foraminal decompression for failed back surgery syndrome under local anesthesia. Int J Spine Surg 2014.8. [PubMed]
- McGrath LB Jr, Madhavan K, Chieng LO, et al. Early experience with endoscopic revision of lumbar spinal fusions. Neurosurg Focus 2016;40. [Crossref] [PubMed]
- Ahn Y, Oh HK, Kim H, et al. Percutaneous endoscopic lumbar foraminotomy: an advanced surgical technique and clinical outcomes. Neurosurgery 2014;75:124-33; discussion 132-3. [Crossref] [PubMed]
- Sairyo K, Higashino K, Yamashita K, et al. A new concept of transforaminal ventral facetectomy including simultaneous decompression of foraminal and lateral recess stenosis: Technical considerations in a fresh cadaver model and a literature review. J Med Invest 2017;64:1-6. [Crossref] [PubMed]


