
Utility of the claw sign in spine magnetic nuclear resonance with diffusion to differentiate Modic type I changes for degenerative disease versus infection
Introduction
In 1988, Modic and his colleagues described changes in the subchondral bone marrow of the vertebral plates in patients with degenerative disease or other pathologies, which were observed in the nuclear magnetic resonance (NMR) of the spine and were subdivided into three patterns of changes of signal, called Modic type I, Modic type II and Modic type III (1).
Histologically, the Modic type I changes of the vertebral plates show an active inflammatory state that coincide with the disruption and fissures in the vertebral plates and vascular granulation tissue in the bone marrow. Modic type II changes correlate with replacement of fat marrow. These Modic changes are dynamic; a type I Modic can be converted into a type II Modic and vice versa (1).
The main differential diagnosis of the Modic type I changes of the vertebral plates due to degenerative disease in column NMR is the infection in its early stages, since its findings are similar to Modic type I changes due to degenerative pathology (2). A great erosion of the vertebral plates, associated with extra-spinal inflammation, tilts the diagnosis toward infection, but does not exclude other diagnoses (Figure 1) (2).
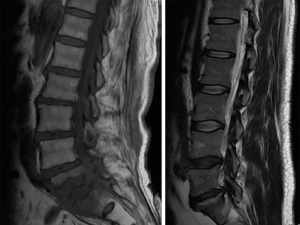
Recently, the use of diffusion in the MRI of Spine, has shown an increase in its use in the diagnosis of spinal pathology, although it is not widely used in the spine and is not well stablished in protocols for its routine performance (3,4). Also it is beginning to have great value in the evaluation of some pathological processes such as tumors and infections (5,6). Modic type I degenerative changes in conventional magnetic resonance can often simulate or suggest infection that leads to the performance of additional costly and sometimes invasive diagnostic methods (7,8). Characteristically in conventional magnetic resonance images we can observe infectious processes as an increase in the signal intensity in the T2 sequences, loss of the intranuclear cleft, peripheral enhancement of the affected disc with the contrast medium and irregularity of the vertebral plates (9). The diffusion-weighted image today plays a unique role among imaging techniques, since they provide physiological tissue information (10).
Patel and collaborators in their study in 2014 using a protocol and the concept of diffusion in column MRI, could demonstrate that by means of the “claw sign” (see Figure 2) which is identified as combined traces in diffusion images, well defined, marginal, linear, usually paired with signal of high intensity, located within the adjacent vertebral bodies between the vascularized bone marrow, near the affected disc, which is supposed to represent a form of physiological reactive response or induration, it can de differentiate a degenerative disease with Modic type I changes from an infection (11).
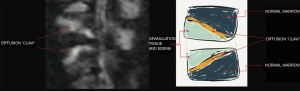
In this series of cases, the algorithm used by Patel et al. was implemented. And the results are described below.
Methods
This is a case series study, where the clinical records of patients who consulted the emergency department for lumbar pain without clear etiology, between January 1, 2017 and December 31, 2017, were analyzed. All subjects were evaluated in Neurosurgery service.
Due to axial lumbar pain, subjects were hospitalized and studies were ordered, including contrasted MRI of the lumbosacral spine. Then, with laboratory tests and Modic type I changes in MRI findings, it was not possible to differentiate between degenerative disease vs. spondylodiscitis, so the algorithm used in the Patel and collaborators study was applied (see Figure 3).
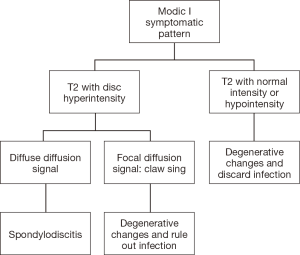
Descriptive statistics, frequencies and proportions were made for the categorical variables and for the measured numerical variables of central tendency and standard deviation or position measurements when their distribution was not normal. The statistical analysis was performed in SPSS Statistics 24.
It does not require any approval of the ethics committee, since it is a study which only followed they; the patients were not intervened by us.
Results
Patients, who were admitted at emergencies, were followed up between January 1, 2017 and December 31, 2017, identifying 13 patients with lumbar or dorsal pain over 3 months of evolution, with nonspecific symptoms, which were very difficult to differentiate between degenerative disease and infection. Of the 13 patients identified, 5 patients (38.46%) were female and 8 patients (61.54%) were male. The age in male patients was between 21 and 74 years, with an average age of 49.87 years, unlike in women who were between 37 and 78 years, with an average age of 62.2 years.
Regarding the previous and pathological history, 5 patients (38.46%) reported arterial hypertension, 4 patients (30.77%) diabetes mellitus, 4 patients (30.77%) chronic kidney disease stage V in management with hemodialysis, 3 patients (23.08%) presented immunosuppressive conditions and 3 patients (23.08%) had a history of spinal surgery with instrumentation.
To these hospitalized patients, laboratory tests were performed, including acute phase reactants. In addition, due to the difficulty in differentiating the cause of the axial lumbar pain, MRI of the Lumbosacral and Thoracic Spine was performed, identifying Modic type I changes, which could not be differentiated from a degenerative disease or an infectious process. Consequently, a complementary study of contrast-enhanced MRI with diffusion and ADC was performed.
Of the 13 cases studied for low back pain, 7 patients (53.85%) confirmed changes of Modic type I due to degenerative disease related to claw signs in spinal MRI diffusion and 6 patients (46.15%) (Figure 4) Modic type I by infection due to absence of claw sign in spinal NMR diffusion.
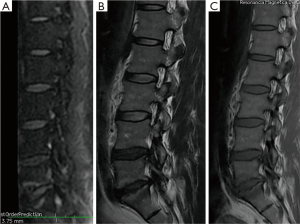
Of the 6 patients (46.15%) with Modic type I changes (Figure 5) due to infection in the absence of claw sign in spinal MRI diffusion, all had infection that was confirmed through blood culture, or biopsy. In five patients (83.33%), who had high acute phase reactants, positive blood cultures were found, isolating germs such as: methinosensitive staphylococcus aureus, streptococcus agalactiae, methinosensitive staphylococcus, pseudomonas aeruginosa, and in immunosuppressed patient’s pseudomonas aeruginosa, multisensible staphylococcus epidermidis, and achromobacter xylosoxidans were isolated. The patients with negative blood cultures (16.66%) required biopsy guided by computerized axial tomography to confirm the initial diagnosis, resulting positive for infection.
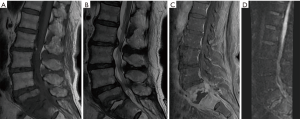
From the findings described above, it was observed that 100% of these patients with absence of claw sign in the diffusion of the spine MRI, had confirmed infection with the complementary examinations.
The 7 patients with Modic type I changes due to degenerative disease with the presence of claw sign in spinal MRI diffusion (Figures 4,6), were followed at 4 weeks and 3 months by external consultation, performing laboratory tests and images of control, where 100% of the patients had no changes observed compared with the previously findings and there were no imaging changes in comparison to their emergency visit, except for pain relief with analgesics and physical therapy.
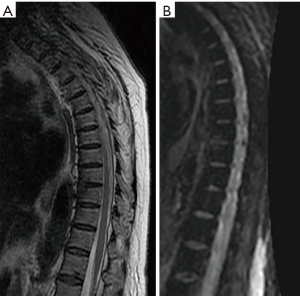
Discussion
Chronic axial lumbar pain continues to be a problem, for both, its diagnosis and for its treatment (12). Although the prevalence of axial lumbar pain is high, due to the involvement of the facet and disc joints, it has also been shown that the vertebral plates are richly innervated and that these affected plates can cause lumbar pain (13).
Anatomically, vertebral body plates are structures that have a bone and cartilaginous components (14). Its cartilaginous part is formed by proteoglycan molecules, reformed with collagen fibers and chondrocytic cells, which is in intimate contact with the inner ring of the vertebral disc (15). In the bone component there are sinusoidal and nerve cells, forming a bone-disk interface (16). This capillary and nervous network enters through the basis- vertebral formations of the posterior cortex of the vertebral bodies, through which the basis- vertebral vascular network of the entire vertebral body also enters (17).
It has several functions, one of them is the nutritional supplement of the vertebral disc, through the external layer of the fibrous ring of the disc, by the diffusion mechanism, nourishes the plates to the intervertebral disc (18). In addition, they also absorb a considerable hydrostatic pressure resulting from the mechanical loading of the spine, a function that is also supported by the vertebral disc (19).
Additional studies have identified nerve fibers and blood vessels in the vertebral plates and subchondral bone, predominantly in degenerative disc disease, suggesting that they may be associated with low back pain (18).
The most common defect found in the vertebral plates, are Schmorl’s nodules (Figure 7), which are a vertical protrusion of the vertebral disc nucleus content, in the vertebral body, which are observed in more than 70% of the vertebral spine, above 50 years, and indicate that the intervertebral discs with Schmorl’s nodules have more degenerative disease than those that do not have them (20)
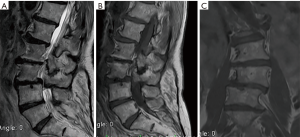
The damage in the vertebral plates, occurs more frequently in the upper plate (Figure 5) and in the central region, which are the thinner and weaker areas, this is exacerbated with age, making the central zone more porous (17). In addition, this disruption affects the transport of nutrients to the intervertebral disc, prompting the inflammatory response of the disc or vertebra (21).
Many of the axial lumbar pains do not require images, however if the pain persists for more than 4 weeks despite medical management, it requires an imaging study. There are causes in which images are indicated in the initial approach, such as lumbar pain associated with radiculopathy, narrow spinal canal, cauda equina syndrome, neurological deficit and infection (22).
A not very common cause of chronic low back pain, is spondylodiscitis, which is a combination of an inflammatory process, which affects one or more vertebral bodies (spondylitis) with subsequent involvement of the intervertebral disc (discitis) and finally adjacent nerve structures (Figure 8). Its presentation increases with age, but there are other risk factors, such as surgery, kidney disease, alcohol and drug abuse, diabetes and ultimately HIV (23). Comparing with this series of cases, it was observed that several patients had these risk factors to develop spondylodiscitis.
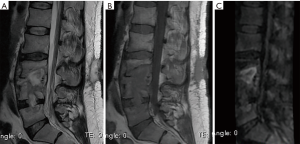
Spondylodiscitis is estimated to be 2% to 7% of all cases of vertebral osteomyelitis, presenting two age peaks of presentation, under 20 years of age and a range between 50 and 70 years, where the most frequent isolated germ is staphylococcus aureus (24).
For the diagnosis of spondylodiscitis, column NMR offers great value. It allows the visualization of infectious changes in several sites and associated to the contrast means it allows to observe if there is uptake and if it presents collections. Conventional column NMR in the T1 sequence has a weak signal by the affected vertebral body, and destruction of the cartilaginous surface of the vertebral bodies and the intervertebral disc, and in the T2 sequence a strong signal in the body part and affected disk (25,26).
Independent studies suggest that the changes adjacent to Modic type I and type II changes, which are observed in the MRI of the spine, agree with the discogenic pain. In a prospective study, the severe Modic type I and type II abnormalities of the vertebral plates correlated 100% with the discogenic pain at that level (27).
The precise etiology of the changes observed in the MRI of the spine in the bone marrow is not very well understood, but it seems to be an autoimmune component and an inflammatory response to the chemicals produced by the dead cells (17).
Taking into account that for a conclusive diagnosis in spondylodiscitis or vertebral osteomyelitis, it requires biopsy and culture, which are invasive and not always definitive, an attempt was made to describe the usefulness of a pattern such as the “claw sign” to confirm the presence of true degenerative changes in the vertebral plates and reduce the concern of a possible spondylodiscitis or vertebral osteomyelitis according to the K.B. Patel and collaborators (11).
The patients who generate a greater diagnostic challenge are those who have a high suspicion of imaging with signal intensities that are very similar to Modic type I changes associated with hyperintensity changes in the intervertebral disc in the spine MRI in the sequence T2 and sometimes an enhancement of the vertebral plate or disc which are generated by the high vascularization of the bone marrow and edema (1). They are usually patients who have no clinical suspicion of infection, and the lack of laboratory data suggesting an infectious process, including negative blood cultures. In several patients we can see that although it is not possible to exclude an infectious process, based on the patient's clinic, therefore, no treatment is offered and we can see in the follow-up images that reveal minimal changes, resolution or evolution to Modic type II changes (11).
The claw sing is considered absent if the findings in diffusion are diffuse and are not well defined, seems logical in a gradual and progressive process. The degenerative disease of the intervertebral disc produces a well-defined border response. A destructive process such as an infection can progress too quickly and infiltrate diffusely with infiltrated pathogens or edema and not produce a well-defined response in the border area (11).
In addition, it must be taken into account, that the highlight with the disc contrast medium and the vertebral plates provides indeterminate findings, as has been reported in several studies (7). At least some highlight can be observed in some cases in the vertebral plates, but it is not very helpful in differentiating the infectious processes from the degenerative pathology, and it can only be observed between 25–30% of the cases of spondylodiscitis and from 11% to 17% of the cases of degenerative pathology (11).
In the study by Patel and colleagues, 73 patients who were identified had characteristics similar to Modic type I changes and were divided into 3 groups: 33 patients with Modic type I changes, 22 patients with confirmed infection and 20 patients with suspicion of infection (11). Unlike in our study, which was followed up for 1 year, 13 patients were identified, which were classified as Modic type I changes due to degenerative disease and infection.
In the study by Patel and colleagues, 2 neuroradiologists were used, which, when the claw sign was identified, in 38 of 39 patients (97%) and 29 of 29 patients (100%) were found to be free of infection and when the sign of claw was absent 17 of 17 patients (100%) and 13 of 14 patients (93%) showed infection (11). Comparing these results with our study, of the 13 cases identified by lumbar pain, 7 patients (53.85%) were confirmed findings of Modic type I changes due to degenerative disease having a claw sign in the spinal MRI diffusion, and 6 patients (46.15%) Modic type I changes due to infection in the absence of claw sign in spinal NMR diffusion. Finally, confirming it with laboratory studies with blood cultures and direct samples, the 6 cases of the patients (100%) with spinal MRI with diffusion with absent claw sing were confirmed with infection.
Conclusions
MRI of spinal column with diffusion is useful to differentiate patients with type I changes due to degenerative disease with positive claw sign, of patients with type I changes due to infection with absent claw sign.
In addition, in patients with compromised renal function, Column MRI with diffusion without contrast could be a diagnostic alternative, since it does not require contrast media to confirm infection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: It does not require any approval of the ethics committee, since it is a study which only followed they; the patients were not intervened by us.
References
- Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193-9. [Crossref] [PubMed]
- Crockett MT, Kelly BS, van Baarsel S, et al. Modic Type 1 Vertebral Endplate Changes: Injury, Inflammation, or Infection? AJR Am J Roentgenol 2017;209:167-70. [Crossref] [PubMed]
- Castillo M, Arbelaez A, Smith JK, et al. Diffusion-weighted MR imaging offers no advantage over routine noncontrast MR imaging in the detection of vertebral metastases. AJNR Am J Neuroradiol 2000;21:948-53. [PubMed]
- Castillo M. Diffusion-weighted imaging of the spine: is it reliable? AJNR Am J Neuroradiol 2003;24:1251-3. [PubMed]
- Dietrich O, Biffar A, Reiser MF, et al. Diffusion-weighted imaging of bone marrow. Semin Musculoskelet Radiol 2009;13:134-44. [Crossref] [PubMed]
- Eastwood JD, Vollmer RT, Provenzale JM. Diffusion-weighted imaging in a patient with vertebral and epidural abscesses. AJNR Am J Neuroradiol 2002;23:496-8. [PubMed]
- Oztekin O, Calli C, Kitis O, et al. Reliability of diffusion weighted MR imaging in differentiating degenerative and infectious end plate changes. Radiol Oncol 2010;44:97-102. [Crossref] [PubMed]
- Dihlmann W. Hemispherical spondylosclerosis--a polyetiologic syndrome. Skeletal Radiol 1981;7:99-106. [Crossref] [PubMed]
- Maiuri F, Iaconetta G, Gallicchio B, et al. Spondylodiscitis. Clinical and magnetic resonance diagnosis. Spine (Phila Pa 1976) 1997;22:1741-6. [Crossref] [PubMed]
- Moseley ME, Wendland MF, Kucharczyk J. Magnetic resonance imaging of diffusion and perfusion. Top Magn Reson Imaging 1991;3:50-67. [PubMed]
- Patel KB, Poplawski MM, Pawha PS, et al. Diffusion-weighted MRI "claw sign" improves differentiation of infectious from degenerative Modic type 1 signal changes of the spine. AJNR Am J Neuroradiol 2014;35:1647-52. [Crossref] [PubMed]
- Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med 2009;169:251-8. [Crossref] [PubMed]
- van Dieën JH, Weinans H, Toussaint HM. Fractures of the lumbar vertebral endplate in the etiology of low back pain: a hypothesis on the causative role of spinal compression in aspecific low back pain. Med Hypotheses 1999;53:246-52. [Crossref] [PubMed]
- Taylor JR. Growth of human intervertebral discs and vertebral bodies. J Anat 1975;120:49-68. [PubMed]
- Ghosh P, Hukins DWL. Disc structure and function: The biology of the intervertebral disc. Florida, Boca Raton: CRC Press, 1988:1-37.
- Bailey JF, Liebenberg E, Degmetich S, et al. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat 2011;218:263-70. [Crossref] [PubMed]
- Lotz JC, Fields AJ, Liebenberg EC. The role of the vertebral end plate in low back pain. Global Spine J 2013;3:153-64. [Crossref] [PubMed]
- Moore RJ. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J 2006;15 Suppl 3:S333-7. [Crossref] [PubMed]
- Broberg KB. On the mechanical behaviour of intervertebral discs. Spine (Phila Pa 1976) 1983;8:151-65. [Crossref] [PubMed]
- Vernon-Roberts B, Pirie CJ. Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol Rehabil 1977;16:13-21. [Crossref] [PubMed]
- Bisla RS, Marchisello PJ, Lockshin MD, et al. Auto-immunological basis of disk degeneration. Clin Orthop Relat Res 1976.205-11. [PubMed]
- Clarençon F, Law-Ye B, Bienvenot P, et al. The Degenerative Spine. Magn Reson Imaging Clin N Am 2016;24:495-513. [Crossref] [PubMed]
- Fantoni M, Trecarichi EM, Rossi B, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci 2012;16 Suppl 2:2-7. [PubMed]
- Petkova AS, Zhelyazkov CB, Kitov BD. Spontaneous Spondylodiscitis - Epidemiology, Clinical Features, Diagnosis and Treatment. Folia Med (Plovdiv) 2017;59:254-60. [Crossref] [PubMed]
- Endean A, Palmer KT, Coggon D. Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: a systematic review. Spine (Phila Pa 1976) 2011;36:160-9. [Crossref] [PubMed]
- Behre I, Cramer J, Haase N, et al. Spondylitis und Spondylodiszitis. Trauma und Berufskrankheit 2003;5:336-41. [Crossref]
- Weishaupt D, Zanetti M, Hodler J, et al. Painful Lumbar Disk Derangement: Relevance of Endplate Abnormalities at MR Imaging. Radiology 2001;218:420-7. [Crossref] [PubMed]


