
An epidural steroid injection in the 6 months preceding a lumbar decompression without fusion predisposes patients to post-operative infections
Introduction
Lumbar epidural steroid injections (LESI) are commonly used for lumbar radicular complaints as a therapeutic and diagnostic modality. LESI have increased exponentially in the Medicare population since the 2000s (1-3). These procedures are typically used as a treatment option for lumbar radiculopathy prior to surgical intervention. Failure of improvement in symptoms or deteriorating neurological function following epidural injections usually warrants surgical consideration.
Post-operative infections following lumbar decompression without the use of instrumentation are thought to be low with rates ranging from 0.7% to 2.4% (4,5). There have been conflicting studies on risk of epidural injections to post-operative infections in lumbar decompression (6,7). There are also no studies to our knowledge that evaluate the association of LESI and readmission following lumbar decompression.
The purpose of this study was to determine if a recent history of spinal epidural injections predisposed patients to post-operative infections following non-instrumented lumbar decompression. We also sought to determine if there are certain time intervals that can be established as being at higher risk so that practitioners can appropriately counsel patients regarding the risks of injections and subsequent decompressions.
Methods
Data source
A thorough evaluation of the PearlDiver database (PearlDiver Technologies, West Conshohocken, PA, USA) was performed for patients undergoing lumbar decompression for spinal stenosis or disc herniation without instrumentation from 2005–2014. PearlDriver is a privately owned dataset containing a full sample of Medicare data. This data set contains various interventions and conditions based on current procedure terminology (CPT) codes and International Classification of Diseases 9th Revision Clinical Modification Diagnoses and Procedures (ICD-9-CM). All of the data is anonymous and de-identified, therefore no institutional review board is required. Medicare was the nationwide insurance provider for our patient selection.
Patient selection
Inclusion parameters were those having undergone primary lumbar decompression for spinal stenosis or disc herniation without instrumentation (CPT: 63030, 63047). Patients undergoing lumbar fusion procedures (CPT: 22612, 22614, 22633, 22630, 22830), and revision lumbar procedures (CPT: 22830, 63042, 63044) were excluded. Data for patients who underwent LESI was also queried (CPT: 64483, 62311). Ninety-day postoperative infection was assessed using ICD-9 codes (998.5, 998.51, 998.59, 996.67) and CPT codes (20005, 22015) (Table S1).

Full table
Matching
Utilizing Boolean operations, a control group was created for comparison purposes, which included all patients meeting the above procedural criteria without a previous documented LESI. This control group was matched to the 3 study cohorts by using the maximum number of available patients with a similar distribution of variables: age, gender, race, region and comorbidities such as hypertension, auto immune deficiency, body mass index, chronic kidney disease, diabetes, chronic liver disease, chronic obstructive pulmonary disease, hyperlipidemia, cardiac disease, and tobacco use. Matching was performed strictly on a one-to-one basis, where for every patient in the cohort study, one patient in the control group was selected. The matched cohorts were then assessed by the average Charlson-Comorbidity Index (CCI) to ensure adequate matching. Matching with the CCI allowed for a more accurate comparison between the two groups.
Data analysis
Statistical comparisons of cohort demographics and postoperative infection rates between the study and control groups were performed using Pearson chi-square analysis. Odds ratios and their 95% confidence intervals were calculated. For all statistical comparisons, P<0.05 was considered significant. SPSS software (version 22 for Macintosh, IBM) was used for all statistical calculations.
Results
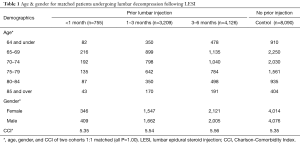
The database was queried for patients undergoing primary decompression without fusion, identifying 4 groups: (I) lumbar decompression with no 6-month LESI history (n=8,090); (II) lumbar decompression performed within 0–1 month after LESI (n=755); (III) lumbar decompression between 1 and 3 months after LESI (n=3,209); (IV) lumbar decompression performed between 3 and 6 months after LESI (n=4,126). The study groups each had a P value of 1.00 compared to the control group (n=8,090), indicating no difference was found between the study and control groups (Table 1).
Full table
The rate of post-operative infection was low in all time periods, ranging from 1.98% to 1.40%. Patients having LDC within a month after their LESI had greater odds of infections (OR =1.37; 95% CI, 0.62–3.00; P=0.43), but no statistical significance was found in this group compared to the matched control group. In the LDC 1 to 3 months following a LESI, there was a statistically significant higher incidence of 90-day postoperative infection compared to the matched control group (OR =4.69; P<0.001). Similarly, patients who had a LDC 3 to 6 months following LESI had a statistically significant higher incidence of infection compared to the matched control group (OR =5.33; 95% CI, 2.79–10.17; P<0.001) (Table 2).

Full table
Discussion
The association of intra-articular corticosteroid injections and surgical site infection has been well documented in other orthopaedic subspecialties (8-11). While there are some existing studies evaluating LESI and infection rates following spine surgery, these studies have shown conflicting results regarding the correlation between LESI and risk of post-operative infection with various spine procedures (6,7,12). Eismont et al. used a canine model undergoing serial epidural steroid injections to compare the impact of corticosteroids on dural tissue (13). In this study they demonstrated a 27% decrease in dural tensile strength as well as a 72% decrease in mitochondria volume in those canine given serial epidural injections compared to the control group. This current study illustrates a statistically significant increased risk of post-operative infection when LESI was placed 1–6 months prior to a primary single level lumbar decompression without fusion. Fortunately, the overall incidence of infection for this specific cohort remained low (1.4–1.98%).
The use of epidural injections for lumbar radiculopathy is relatively safe. A recent study of 52,935 patients who received LESI reported major complications in only 6 patients (0.011%), 4 of which developed infection, and 2 developed hematomas (14). Despite the low incidence of infection following epidural steroid injection, multiple case reports have shown possible devastating complications, including extensive spinal abscesses, discitis, and death (15-20). These infections are theorized to occur for various reasons. First, direct contamination or inoculation from skin flora may lead to infection. Secondly, glucocorticosteroids function by directly or indirectly reducing the inflammation process, which can limit the immunological response to indolent or early infection (21). Singla et al. suggests a critical time period may exist before the immunosuppressive properties of glucocorticoids resolve (12). The pharmacokinetics of these theories have not been further studied. It is reasonable to believe that these processes could be active for months when considering patients who receive these injections report relief of symptoms due to glucocorticoids for months, indicating the glucocorticoids may be active in this space (12). This group also suggests the glucocorticoids prevent the natural host response to tissue injury and pathogen exposure, which leads to increased susceptibility to infection (12). It is also proposed that epidural injections can lead to epidural scarring, increased vascularization and promotion of degenerative changes at the injection site, which can potentially complicate the surgical site also (22). These multifactorial concerns all may contribute to the increased risk of surgical site infection. Though the anti-inflammatory properties of steroids may be advantageous in the pre-operative period and relieve patients of their symptoms, in the post-operative period, the anti-inflammatory properties may be detrimental.
LESI as a risk factor may be confounded by the length of surgery due to severe stenosis (9,12). It can be postulated our cohort that required a LESI prior to a subsequent spinal decompression was more likely to have a more severe stenosis that was not able to be relieved with injections or postponed beyond 6 months. An explanation for the increased infection rates in the LESI 1–6 months cohort is that potentially these patients would require slightly more decompression and an increased length of surgery since operative time is another known risk factor for surgical site infection (23).
An inevitable limitation in this study is the use of an insurance database which requires accurate human input, and so there is the potential for missed and incorrect data. Additionally, we were unable to account for the amount of previous LESIs which may predispose the patient to additional infection risks or indicate more severe stenosis. This dataset also does not indicate the length of surgery or post-operative discharge disposition which also could indicate the complexity of the case or patient condition. It is also feasible that LESIs cause indwelling latent infections that are not included in a review of the 90-day post-operative course. Lastly, while a multivariate analysis of comorbidities was not possible, we accounted for this difference in comorbidities with the use a matched control group based on CCI (24,25).
Conclusions
While LESI is helpful for possibly delaying or avoid lumbar surgery, it may predispose patients to higher infection and readmission rates following lumbar decompressions without fusion. This study provides additional evidence for the increased risk of post-operative infection in lumbar decompression performed after LESI. It also proves evidence for an increased risk of readmission following lumbar decompression after LESI. Surgeons and pain management specialist should counsel patients on these risks and possibly modify the time to surgical intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Manchikanti L, Pampati V, Hirsch JA. Utilization of Interventional Techniques in Managing Chronic Pain In Medicare Population from 2000 to 2014: An Analysis of Patterns of Utilization. Pain Physician 2016;19:E531-46. [PubMed]
- Manchikanti L, Pampati V, Falco FJ, et al. Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Physician 2013;16:E349-64. [PubMed]
- Manchikanti L, Pampati V, Boswell MV, et al. Analysis of the growth of epidural injections and costs in the Medicare population: a comparative evaluation of 1997, 2002, and 2006 data. Pain Physician 2010;13:199-212. [PubMed]
- Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2011;36:556-63. [Crossref] [PubMed]
- Gruskay J, Kepler C, Smith J, et al. Is surgical case order associated with increased infection rate after spine surgery? Spine (Phila Pa 1976) 2012;37:1170-4. [Crossref] [PubMed]
- Yang S, Werner BC, Cancienne JM, et al. Preoperative epidural injections are associated with increased risk of infection after single-level lumbar decompression. Spine J 2016;16:191-6. [Crossref] [PubMed]
- Seavey JG, Balazs GC, Steelman T, et al. The effect of preoperative lumbar epidural corticosteroid injection on postoperative infection rate in patients undergoing single-level lumbar decompression. Spine J 2017;17:1209-14. [Crossref] [PubMed]
- Werner BC, Cancienne JM, Burrus MT, et al. The timing of elective shoulder surgery after shoulder injection affects postoperative infection risk in Medicare patients. J Shoulder Elbow Surg 2016;25:390-7. [Crossref] [PubMed]
- Cancienne JM, Gwathmey FW, Werner BC. Intraoperative Corticosteroid Injection at the Time of Knee Arthroscopy Is Associated With Increased Postoperative Infection Rates in a Large Medicare Population. Arthroscopy 2016;32:90-5. [Crossref] [PubMed]
- Werner BC, Cancienne JM, Browne JA. The Timing of Total Hip Arthroplasty After Intraarticular Hip Injection Affects Postoperative Infection Risk. J Arthroplasty 2016;31:820-3. [Crossref] [PubMed]
- Werner BC, Cancienne JM, Burrus MT, et al. Risk of Infection After Intra-articular Steroid Injection at the Time of Ankle Arthroscopy in a Medicare Population. Arthroscopy 2016;32:350-4. [Crossref] [PubMed]
- Singla A, Yang S, Werner BC, et al. The impact of preoperative epidural injections on postoperative infection in lumbar fusion surgery. J Neurosurg Spine 2017;26:645-9. [Crossref] [PubMed]
- Slucky AV, Sacks MS, Pallares VS, et al. Effects of epidural steroids on lumbar dura material properties. J Spinal Disord 1999;12:331-40. [Crossref] [PubMed]
- Lee JW, Lee E, Lee GY, et al. Epidural steroid injection-related events requiring hospitalisation or emergency room visits among 52,935 procedures performed at a single centre. Eur Radiol 2018;28:418-27. [Crossref] [PubMed]
- Gotz F, Lanfermann H, Becker H. Cervical epidural abscess following lumbar epidural steroid injections. Klin Neuroradiol 2009;19:220-6. [PubMed]
- Hoelzer BC, Weingarten TN, Hooten WM, et al. Paraspinal abscess complicated by endocarditis following a facet joint injection. Eur J Pain 2008;12:261-5. [Crossref] [PubMed]
- Hooten WM, Mizerak A, Carns PE, et al. Discitis after lumbar epidural corticosteroid injection: a case report and analysis of the case report literature. Pain Med 2006;7:46-51. [Crossref] [PubMed]
- Knight JW, Cordingley JJ, Palazzo MG. Epidural abscess following epidural steroid and local anaesthetic injection. Anaesthesia 1997;52:576-8. [Crossref] [PubMed]
- Kraeutler MJ, Bozzay JD, Walker MP, et al. Spinal subdural abscess following epidural steroid injection. J Neurosurg Spine 2015;22:90-3. [Crossref] [PubMed]
- Lee Y, Kim JS, Kim JY. Cervical Meningomyelitis After Lumbar Epidural Steroid Injection. Ann Rehabil Med 2015;39:504-7. [Crossref] [PubMed]
- McLain RF, Kapural L, Mekhail NA. Epidural steroids for back and leg pain: mechanism of action and efficacy. Cleve Clin J Med 2004;71:961-70. [Crossref] [PubMed]
- Zusman N, Munch JL, Ching A, et al. Preoperative epidural spinal injections increase the risk of surgical wound complications but do not affect overall complication risk or patient-perceived outcomes. J Neurosurg Spine 2015;23:652-5. [Crossref] [PubMed]
- Piper KF, Tomlinson SB, Santangelo G, et al. Risk factors for wound complications following spine surgery. Surg Neurol Int 2017;8:269. [Crossref] [PubMed]
- Fang A, Hu SS, Endres N, et al. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460-5. [Crossref] [PubMed]
- Veeravagu A, Patil CG, Lad SP, et al. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976) 2009;34:1869-72. [Crossref] [PubMed]


