
Cost-utility of revisions for cervical deformity correction warrants minimization of reoperations
Introduction
Surgery for degenerative cervical spine conditions is increasing (1,2). Cervical deformity (CD) surgery is a subset of these cases and follows a similar epidemiological trend. A recent cost-utility analysis showed the average Medicare reimbursement (cost) per CD surgery to be just over $55,000 dollars (3). As healthcare spending increases per capita and as a function of the GDP, it becomes increasingly necessary to critically examine the cost and cost-benefit ratio of interventions.
CD surgeries are technically demanding and require significant planning relating to the patient’s pre-operative functional status, global and cervical alignment, and comorbidities. When clinically indicated, these surgeries can result in dramatic improvement in neurologic functionality and quality of life (4,5). Despite the meticulous planning there is a risk of revision surgery. Reasons for revision surgery after cervical spine reconstruction include infection, kyphotic deformity, distal junctional kyphosis (DJK), residual or recurrent neurological symptoms, adjacent segment disease, and pseudoarthrosis (6).
An accepted method for calculating the cost-effectiveness of an intervention is to establish the change in quality of life before and after the intervention using a general or disease specific validated questionnaire, and then calculating the cost for this intervention relative to a normalized unit gain in quality of life (3). The questionnaires can be general quality of life measures such as the EuroQol Five-Dimensions questionnaire (EQ-5D), or they can be disease specific metrics such as the Neck Disability Index (NDI). It can be difficult to accurately estimate the cost of an intervention such as spine surgery as there are numerous variables involved. However, one accepted method is to use total Medicare disbursement fees as a surrogate for the total cost of the intervention.
The purpose of this study is to calculate the cost per quality-adjusted life year (QALY) after CD revision surgery, using Medicare reimbursement. Medicare reimbursements are widely accepted as a surrogate estimate for the cost of a given intervention, and allow for comparison across medical disciplines (7).
Methods
Data source
This study is a retrospective review of a prospectively-collected database of CD patients enrolled from 13 sites within the United States. Internal Review Board approval was obtained at the submitting site (No. S12-02939) and each participating site prior to study initiation and informed consent was given by each included patient. Inclusion criteria for the database were patients ages ≥18 years, and radiographic evidence of CD at baseline assessment, defined as the presence of at least 1 of the following: cervical kyphosis (C2–7 Cobb angle >10°), cervical scoliosis (C2–7 coronal Cobb angle >10°), C2–7 sagittal vertical axis (cSVA) >4 cm, or chin-brow vertical angle (CBVA) >25°. CD patients meeting radiographic inclusion with available baseline and 1-year follow-up data were included in this study. Patients with active tumors or infections were excluded from the study.
Data collection
Demographic and clinical data collected included patient age, sex, body mass index (BMI), prior cervical surgery, and Charlson Comorbidity Index (CCI). Surgical data collected included operative time, estimated blood loss, surgical approach, off-label use of bone morphogenetic protein 2 (BMP-2), osteotomy use and number of osteotomies, levels fused, and instrumentation used.
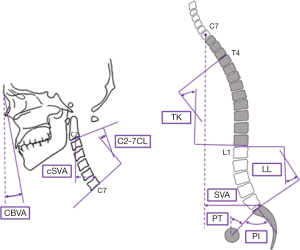
Patients were evaluated using full-length free-standing lateral spine radiographs (36" long-cassette) at baseline and 1-year post-operative follow-up visit. Radiographs were analyzed using dedicated and validated software (SpineView®; ENSAM, Laboratory of Biomechanics, Paris, France) at a single center with standard techniques (8-10). Measured cervical spine parameters included cSVA (offset from the C2 plumbline and the posterosuperior corner of C7), C2–C7 lordosis [cervical lordosis (CL): Cobb angle between C2 inferior endplate and C7 inferior endplate], T1 slope minus CL (TS-CL: mismatch between T1 slope and CL), and CBVA (angle subtended between the vertical line and the line from the brow to the chin). C2–T3 lordosis (sagittal Cobb angle between C2 inferior endplate and T3 inferior endplate), C2–T3 SVA (offset from C2 plumbline and T3 inferior endplate), and C2 angle (subtended between upper endplate of C2 and the horizontal). Measured spinopelvic parameters (Figure 1) included: sagittal vertical axis (SVA: C7 plumb line relative to the posterior-superior corner of S1), pelvic incidence minus lumbar lordosis (PI-LL: mismatch between pelvic incidence and lumbar lordosis), and pelvic tilt (PT: angle between the vertical and the line through the sacral midpoint to the center of the two femoral heads).
Cost calculations
The PearlDiver database, which gives 1-year Medicare reimbursement rates, was used to calculate costs using job order cost accounting (“charge analysis”). Each CD surgical procedure in the database used in this study was retrospectively assigned ICD-9 codes for both the primary surgery and any subsequent revision procedures (e.g., posterior cervical refusion 4–8 level: 81.33, 81.63; full list in Table 1). Medicare 1-year reimbursements from on the PearlDiver database were queried for those combinations of ICD-9 codes for each patient’s procedures, and the average Medicare 1-year reimbursement was assigned. One-year reimbursement represents how much Medicare contributed to the hospital to cover all procedures until day 30, including the cost of postoperative complications, management in a follow-up clinic, and readmissions. Cervical procedures queried included: posterior cervical fusion (2–3 level; 4–8 level; or 9+ level) and anterior cervical fusion (2–3 level, 4–8 level, or 9+ level) for index and revision procedures.

Full table
Utility calculation
QALY was used to measure the quality of care (3,11). The QALYs gained were calculated using the following equation {Eq. [1]}:
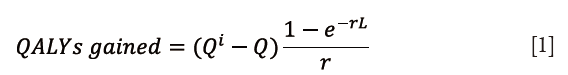
QALY is a measure of health-related quality of life (Q), which calculates the Q while taking into account the life expectancy (L) to determine health benefits, where e is Napier’s mathematical constant and r is the discount rate. World Health Organization (WHO) recommends a discount rate of 3%, which was used in this analysis (12,13). Total utility gained by an intervention was calculated by a change in Q (Qi – Q) and was multiplied by the life expectancy to determine total QALYs gained. Life expectancy was selected manually, based on US national averages for females (81.6 years) and males (76.9 years). QALY was calculated using a general health-state patient-reported quality of life metric, the EQ5D, as well as a questionnaire that is specific to neck-pain related disability, the NDI and the Modified Japanese Orthopedic Association Questionnaire (mJOA) which assesses overall functional status by way of degenerative cervical myelopathy. NDI was mapped to a SF-6D index value in order to translate the values into QALY (3,14,15).
Statistical analysis
Demographic and clinical variables were assessed using chi-squared and t-tests for categorical and continuous variables, respectively. Utility was calculated using both EQ-5D and NDI. Cost (dollars) per QALYs gained was calculated at 1-year post-operatively. Two-sided P values less than 0.05 were considered statistically significant. All statistical analyses were done using SPSS Version 23 (Armonk, NY).
Results
Patient demographics
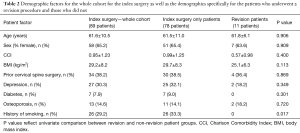
Eighty-nine CD patients were included (61.6 years old, 65.2% female, BMI 29.2 kg/m2, Table 2). The most common diagnoses for these CD patients were degenerative kyphosis (48.2%), stenosis or myelopathy (20.0%), and iatrogenic kyphosis (14.1%). Thirty-point-three percent of patients had depression, 29.2% had a history of smoking, and 14.6% had osteoporosis. Thirty-eight-point-six percent of patients had a prior cervical spine surgery.
Full table
For patients who underwent a revision surgery, the average age was 61.8 years, BMI was 25.1 kg/m2, and CCI was 0.57. Thirty-six-point-four percent of patients who went on to have a revision surgery had prior cervical spine surgery before enrollment.
Surgical details
CD correction for these patients involved a mean 7.7±3.7 levels fused, with 34% combined approach surgeries, 49% posterior-only and 17% anterior-only. Eighteen (20.2%) of cases had a Smith-Peterson osteotomy and 17 (19.1%) of cases had a three-column osteotomy performed. Thirty-three (37.1%) cases used BMP-2. Overall, mean operative time was 525±520 minutes and mean blood loss was 801±933 mL. For patients that did not undergo revision, the median uppermost instrumented vertebra (UIV) and lowermost instrumented vertebra (LIV) following primary surgery were C2 and T2, respectively. For patients that would later require a revision surgery, the median UIV and LIV following primary surgery were C3 and T4, respectively. Following revision, the median UIV and LIV for these patients were C4 and T4, respectively. There were no significant differences between patients who required a reoperation and those who did not in operative time (465 vs. 533 min, respectively, P=0.686) and blood loss (1,392 vs. 730 mL, P=0.075) of index procedure.
Pre- and post-operative radiographic alignment
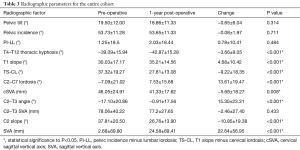
At baseline, there were no significant differences in any radiographic parameters between revision and non-revision patients (all P>0.05). In looking at pre- to post-operative changes in radiographic alignment, the whole cohort improved in TS-CL (37.32° to 27.81°, P<0.001), cSVA (46.05 to 41.33 mm, P=0.008), C2–T3 angle (−17.10° to −0.91°, P<0.001) and saw an increase in global SVA (2.68 to 24.58 mm, P<0.001, Table 3).
Full table
By contrast, patients who required a revision surgery did not achieve significant alignment correction at 1-year post-operatively (from their index surgery) in any major radiographic parameter (all P>0.05, Table 4).
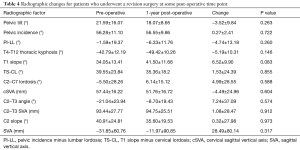
Full table
Health-related quality of life scores
Pre- and post-operative health-related quality of life scores did not differ between patients who later required a revision surgery and those who did not, with the exception of baseline EQ-5D scores (revision: 0.78±0.07, non-revision: 0.73±0.06, P=0.028, Table 5).
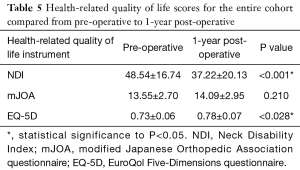
Full table
Revision surgeries
Eleven patients underwent a revision procedure, making the revision rate in this CD cohort 12.4%. Indications for revisions were proximal or DJK (5/11), neurologic impairment [4], infection [1], prominent/painful instrumentation [1]. All revisions were posterior-only surgeries, with an average of 10.3 levels fused (range, 2–27 levels). In looking at the time from the index surgery to the revision procedure, 3 patients underwent a revision in the immediate post-operative period, 1 patient had a revision within 3 months, 3 patients within 6 months and four patients within 1 year of index surgery. Average QALYs gained was 1.62 per revision patient.
Cost analysis
Costs for index surgeries ranged from $20,001–55,205, with the average cost for this cohort of $44,318 and cost per QALY of $27,267 at 1-year follow-up and $37,005 using NDI. Eleven revision surgeries (mean levels fused 10.3) occurred up to 1 year, with an average cost of $41,510. Cost was $28,138 per QALY for reoperations when using EQ-5D. Using NDI mapped to SF-6D to calculate the QALY, the mean cost per QALY for revision surgery was $24,949 gained upon reaching life expectancy and $25,658 when using EQ-5D.
Case examples
Figure 2 displays a 53-year-old female CD patient who underwent a 9-level posterior fusion (~$55,205) and then developed proximal junctional kyphosis (PJK) by 6 weeks post-operatively and underwent an 18-level revision procedure, with an estimated cost of $50,852.
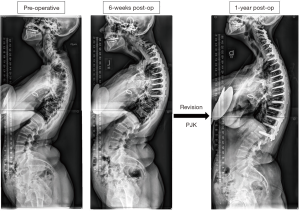
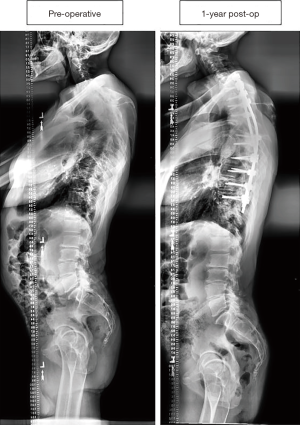
Figure 3 displays a 50-year-old male CD patient who underwent an 11-level posterior fusion, which was estimated at $55,205. This patient did not require a re-operation for any complication.
Discussion
Health-care spending in the USA is currently at over 17% of the gross domestic product and continues to increase as a percentage of the GDP (16,17). While the absolute number is not as high in other Organisation for Economic Co-operation and Development (OECD) countries, the proportion of GDP spent on healthcare has been increasing steadily, and is projected to increase further in the future (16). As the population ages, we anticipate an increase overall demand for healthcare services, with a commensurate increase in costs (18). Thus, it is imperative to assess the return on investment for expensive and resource-heavy interventions such as CD surgery.
Spinal deformity surgery in appropriately selected patients can result in dramatic improvement in quality of life and global functioning (4,5). It is also a costly endeavor due to the complex nature of surgery, the costs associated with hospitalization, and the cost associated with instrumentation. A recent study reported that for index surgery for CD correction, cost per QALY gained up until 1-year follow-up was $646,958 using EQ-5D and that these CD surgeries were within an acceptable range for cost-effectiveness (3). To date, thoracolumbar (TL) spine deformity surgery has been studied in much greater depth than CD surgery. A 4-year follow-up of TL deformity surgery by McCarthy et al. found the total cost of the initial procedure to be $103,143, the total cost at 1 year to be $111,807, and $126,323 at 4-year follow-up (19). A cost-utility analysis by the same group priced the average cost per QALY was $164,261 at 1 year and $154,865 at 2 years for TL deformity surgery (20). Perhaps not unexpectedly, the cost for CD surgery in our cohort was found to be significantly less than the cost quoted in the literature for TL deformity surgery. It would be interesting to further examine where this difference in cost of the index surgery is occurring. In addition, our cost per QALY was lower than previous published literature for CD. However, when analyzing the cost per QALY for a full patient life expectancy, a 5-year life expectancy, and 10-year life expectancy, our cost per QALY was comparable.
Discounting is the mathematical function whereby future health-benefits or outcomes are converted to their present day values (21). A discount rate of 3% which has been commonly used in the CE assessment cervical spine surgery (7,22). It has been previously shown that the spine region specific NDI metric can be accurately mapped to the general health SF-6D index (15,23). The SF-6D index can then be used to obtain QALYs for use in cost-effectiveness studies. We have used the EQ-5D as well as the NDI mapped to SF-6D as the independent variables to calculate QALYs gained, in conjunction with a fixed 3% discount rate that is recommended by the World Health Organization (13).
In our cohort of 89 patients, 11 (12.4%) required revision surgery within 1-year of the index surgery, the majority of which were due to DJK and neurologic impairment. The average cost for revision surgery was $44,310 and the cost per QALY was $27,267. Moreover, the revision surgeries tended to be as large as the primary surgeries with a mean of 10.3 levels fused. Clearly, a revision surgery event has a significant effect on the cost-effectiveness of the primary intervention. DJK was the most common reason for revision in our cohort, and demonstrates the need for further understanding and prevention strategies in order to mitigate the patient and cost burden. The incidence of DJK in the CD population has not been well established. Currently, it is estimated at approximately 24% of CD patients undergoing surgical correction (24,25). It is likely that DJK is the CD corollary problem of PJK in TL deformity surgery. PJK prevention and mitigation techniques should be considered in patients undergoing CD surgery. As a comparator, over a 4-year follow-up period, around 27% of patients undergoing surgery for TL deformity surgery were readmitted for additional spine related intervention after their index surgery (19). Our rate of reoperation was 12.4% over the first year, clearly it remains to be seen if the reoperation rate for CD surgery approaches that of TL deformity over time.
Limitations
We appreciate several limitations. Firstly, the retrospective nature of this study might contribute to site and surgeon variation and bias, though this can also offer increased generalizability of the findings given that the sites are across the continental United States. Secondly, the relatively small sample size and limited follow-up, while limiting, also sets the framework for future studies. The cost data used in this study was derived from a Medicare population, which are potentially older and more comorbid overall in comparison to our study cohort. This might mean that more complications occurred for the Medicare population and thus reimbursement rates were higher. Additionally, Medicare reimbursement rates do not cover expenses that are non-billable. Lastly, as no previous studies have mapped mJOA score to either EQ-5D or SF-6D (both validated metrics used to generate health-state utility scores), we were unable to incorporate mJOA into our economic analysis. Health-state utility scores are necessary for effective economic evaluation, and as such, future research should aim to map mJOA outcomes to the SF-6D to better facilitate cost-effectiveness evaluations for cervical surgery patients with myelopathy.
Conclusions
CD revisions had a cost of $28,138 per QALY, in addition to the $27,267 per QALY for primary CD surgeries. For primary CD patients, CD surgery has the potential to be cost effective, with the caveats that a patient livelihood extends long enough to have the benefits and durability of the surgery is maintained. Efforts in research and surgical technique development should emphasize minimization of reoperation causes just as DJK that significantly affect cost utility of these surgeries to bring cost-utility to an acceptable range.
Acknowledgements
None.
Footnote
Conflicts of Interest: The International Spine Study Group (ISSG) is funded through research grants from DePuy Synthes, and supported the current work. The other authors have no conflicts of interest to declare.
Ethical Statement: Internal Review Board approval was obtained at the submitting site (No. S12-02939) and each participating site prior to study initiation and informed consent was given by each included patient.
References
- Oglesby M, Fineberg SJ, Patel AA, et al. Epidemiological trends in cervical spine surgery for degenerative diseases between 2002 and 2009. Spine (Phila Pa 1976) 2013;38:1226-32. [Crossref] [PubMed]
- McCarthy I, Hostin R, O'Brien M, et al. Health economic analysis of adult deformity surgery. Neurosurg Clin N Am 2013;24:293-304. [Crossref] [PubMed]
- Poorman GW, Passias PG, Qureshi R, et al. Cost-utility analysis of cervical deformity surgeries using 1-year outcome. Spine J 2018;18:1552-7. [Crossref] [PubMed]
- Passias PG, Horn SR, Bortz CA, et al. The Relationship Between Improvements in Myelopathy and Sagittal Realignment in Cervical Deformity Surgery Outcomes. Spine (Phila Pa 1976) 2018;43:1117-24. [Crossref] [PubMed]
- Sabou S, Mehdian H, Pasku D, et al. Health-related quality of life in patients undergoing cervico-thoracic osteotomies for fixed cervico-thoracic kyphosis in patients with ankylosing spondylitis. Eur Spine J 2018;27:1586-92. [Crossref] [PubMed]
- Koerner JD, Kepler CK, Albert TJ. Revision surgery for failed cervical spine reconstruction: review article. HSS J 2015;11:2-8. [Crossref] [PubMed]
- Alvin MD, Miller JA, Lubelski D, et al. Variations in cost calculations in spine surgery cost-effectiveness research. Neurosurg Focus 2014;36:E1. [Crossref] [PubMed]
- Champain S, Benchikh K, Nogier A, et al. Validation of new clinical quantitative analysis software applicable in spine orthopaedic studies. Eur Spine J 2006;15:982-91. [Crossref] [PubMed]
- Rillardon L, Levassor N, Guigui P, et al. Validation of a tool to measure pelvic and spinal parameters of sagittal balance. Rev Chir Orthop Reparatrice Appar Mot 2003;89:218-27. [PubMed]
- O’Brien MF, Kuklo TRTR, Blanke KM, et al. Spinal Deformity Study Group Radiographic Measurement Manual. 2005. Available online: http://www.oref.org/docs/default-source/default-document-library/sdsg-radiographic-measuremnt-manual.pdf?sfvrsn=2
- Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan 2006;21:402-8. [Crossref] [PubMed]
- WHO-CHOICE. Making choices in health: WHO guide to cost-effectiveness analysis. Global Programme on Evidence for Health Policy, World Heal Organ Geneva [Internet]. 2003;71. Available online: http://www.who.int/choice/publications/p_2003_generalised_cea.pdf
- Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ 1994;72:429-45. [PubMed]
- Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409-15. Erratum in: J Manipulative Physiol Ther 1992;15(1):followi. [PubMed]
- Zheng Y, Tang K, Ye L, et al. Mapping the neck disability index to SF-6D in patients with chronic neck pain. Health Qual Life Outcomes 2016;14:21. [Crossref] [PubMed]
- Dieleman JL, Templin T, Sadat N, et al. National spending on health by source for 184 countries between 2013 and 2040. Lancet 2016;387:2521-35. [Crossref] [PubMed]
- OECD (2018), OECD.Stat, (database). Available online: https://data.oecd.org/healthres/health-spending.htm
- Knickman JR, Snell EK. The 2030 problem: caring for aging baby boomers. Health Serv Res 2002;37:849-84. [Crossref] [PubMed]
- McCarthy IM, Hostin RA, Ames CP, et al. Total hospital costs of surgical treatment for adult spinal deformity: an extended follow-up study. Spine J 2014;14:2326-33. [Crossref] [PubMed]
- McCarthy I, O'Brien M, Ames C, et al. Incremental cost-effectiveness of adult spinal deformity surgery: observed quality-adjusted life years with surgery compared with predicted quality-adjusted life years without surgery. Neurosurg Focus 2014;36:E3. [Crossref] [PubMed]
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016;316:1093-103. [Crossref] [PubMed]
- Ament JD, Yang Z, Nunley P, et al. Cost Utility Analysis of the Cervical Artificial Disc vs Fusion for the Treatment of 2-Level Symptomatic Degenerative Disc Disease: 5-Year Follow-up. Neurosurgery 2016;79:135-45. [Crossref] [PubMed]
- Carreon LY, Anderson PA, McDonough CM, et al. Predicting SF-6D utility scores from the neck disability index and numeric rating scales for neck and arm pain. Spine (Phila Pa 1976) 2011;36:490-4. [Crossref] [PubMed]
- Passias PG, Oh C, Horn SR, et al. Predicting the Occurrence of Complications Following Corrective Cervical Deformity Surgery: Analysis of a Prospective Multicenter Database Using Predictive Analytics. Spine J 2017;17:S242-3.
- Passias PG, Vasquez-Montes D, Poorman GW, et al. Predictive model for distal junctional kyphosis after cervical deformity surgery. Spine J 2018. [Epub ahead of print]. [PubMed]


