
An endoscopic surgical technique for treating radiculopathy secondary to S1 nerve compression from a pedicle screw: technical note
Introduction
Pedicle screw instrumentation is used world-wide as an effective means of internal stabilization for spinal arthrodesis. One of the risks of pedicle screw placement is the possibility of the pedicle screw breaching the medial pedicle wall during its placement: this can result in nerve injury or radicular pain and numbness. Although the higher accuracy of navigated pedicle screws has been described in the literature, pedicle screw misplacement even in navigated cases occurs (1-10). The treatment for a patient symptomatic from lumbar radiculopathy secondary to a medially breached pedicle screw is a hardware revision that can often involve a large incision in multilevel fusion cases in order to remove the rod, revise the screw position, and replace the rod. The patient morbidity is significant and the liability to the hospital and surgeon are also significant. Here we present a minimally invasive endoscopic technique for the decompression of an S1 nerve resecting the thread profile and screw edge of a too medially placed S1 pedicle crew utilizing endoscopic drilling. The procedure is performed through a 1-cm incision.
Case report
History and presentation
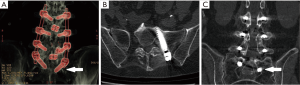
A 60-year-old female patient underwent a lumbar 4–5 transforaminal lumbar interbody fusion (TLIF) in 2011 and had her fusion extended from lumbar 3 to sacral 1 in 2017. Immediately following that fusion surgery and for the next year, she complained of continuous left S1 radicular pain and numbness despite physical therapy and interventional pain management. A CT scan indicated that the left S1 pedicle screw had breached the medial wall of the pedicle and was likely compressing her left S1 nerve (Figure 1). Options for treatment discussed with the patient included revising the screw, removing the screw, and decompressing the S1 nerve by drilling down the screw threads that were impinging on the S1 nerve. The patient sought out a surgeon with experience in endoscopic spine surgery, so opted for a minimally invasive endoscopic approach. The patient underwent an endoscopic interlaminar hemi-laminotomy, medial facetectomy and partial resection of the protruding S1 pedicle screw to decompress and skeletonize the traversing S1 nerve above and below the S1 pedicle. The patient’s radicular symptoms improved immediately, and she remained asymptomatic at the 6-month follow-up.
Operative procedure
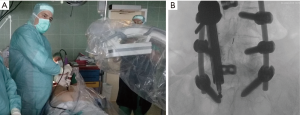
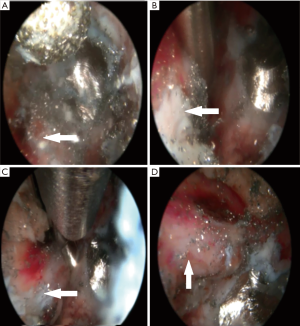
For the endoscopic sacral endoscopic interlaminar hemi-laminotomy, medial facetectomy and partial resection of the protruding S1 pedicle screw the patient was positioned in the prone position on a Kambin frame with flexed hips and knees (Figure 2). The procedure was done under general anesthesia. The Joimax iLESSYS® Delta endoscope was used for the procedure (Figure 2). AP and lateral fluoroscopy were used intermittently throughout the case. A 1-cm incision was made 2 cm left of midline (location determined by fluoroscopy) with a scalpel. Under fluoroscopic guidance, a Jamshidi needle and then sequential dilators were used to target the S1 lamina as a starting point and the final 11.5-mm tubular retractor was inserted. At this point the Joimax® rigid laminoscope with a 10mm outer diameter and 6-mm working channel was inserted through the tubular retractor (Figure 2A,B). Under direct continuous endoscopic visualization, laminar decompression and partial resection of the titanium pedicle screw was achieved using a highspeed endoscopic burr (Joimax®) with a 4.5-mm outer diameter head (Figure 3A). In order to avoid a dural tear, meticulous dissection of the interface between the titanium screw and nerve root was performed with a blunt dissector (Figure 3B). Hemostasis was achieved with a radiofrequency probe. Once titanium screw threads were sufficiently reduced in size by drilling, a lateral recess decompression was performed with a kerrison rongeur decompressing the traversing S1 nerve root along its course over the S1 pedicle (Figure 3C,D). Figure 3 displays the endoscopic camera images from the procedure, and in these images, a large red spot can be seen on the S1 nerve that is likely from the chronic inflammation from the compressed nerve. Once the S1 nerve was completely decompressed, the tubular retractor and endoscope were removed, and the wound was closed with a dissolvable suture.
Postoperative course
The postoperative course was uncomplicated, and the patient’s radicular pain improved immediately after surgery. Six months after his endoscopic procedure, the patient had no clinical symptoms related to the S1 nerve root compression.
Discussion
One interesting feature of the literature on endoscopic spine surgery is how it is used as a rescue procedure for complications associated with many spine surgical procedures: kyphoplasty (11), minimally invasive surgery (MIS) TLIF (12,13), lumbar fusion (14,15), and artificial lumbar disc replacement (16,17). Many advances in spine surgery today are fueled by a rapid influx of new implants. The early phase of clinical studies on spine implants tend to focus on understanding the benefits of these implants. The later phase of clinical studies, after widespread implant adoption and use, then focuses on how to best deal complications that result from these implants. Endoscopic spine surgical approaches certainly offer an interesting minimally invasive approach to dealing with spine implant complications.
There are several drawbacks to consider before embracing endoscopic surgery as the salvage procedure for complications secondary to instrumented spine surgeries. First, there is a learning curve to endoscopic spine surgery. Today, it is uncommon for spine surgeons to be trained in residency or fellowship in endoscopic spine surgery techniques. It is not suggested here that endoscopically exposing and drilling down a pedicle screw be considered by anyone other than a surgeon with significant endoscopic spine surgery experience. Second, the surgery performed here is done with one instrument at a time down the endoscope’s working channel. A retractor is not retracting the nerve while the drill is drilling the pedicle screw. This is a clear disadvantage of a “one instrument at a time” technique. This disadvantage was overcome here by the freedom of angling the endoscope to avoid the nerve and often the continuous irrigation which was used as a gentle dural retractor. If the surgery were performed with a smaller endoscope and beveled tubular retractor, the beveled edge of the tubular retractor could have been used to retract the S1 nerve safely. The larger endoscope used here made it possible to use a larger drill and made drilling out the fusion bone more expeditious.
Conclusions
Minimally invasive endoscopic spine surgery offers many benefits that are attractive to patients: shorter recovery times, small incisions, and less pain. The authors present this technical note for others to consider as a possible minimally invasive solution for the treatment of a radiculopathy caused by a pedicle screw that breached the medial wall of the pedicle. The authors present this technique to make other surgeons aware of the possibility of an endoscopic surgical approach in the case of a hardware complication, but do not suggest this technique be attempted unless the surgeon has significant experience in endoscopic spine surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Mason A, Paulsen R, Babuska JM, et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine 2014;20:196-203. [Crossref] [PubMed]
- Gelalis ID, Paschos NK, Pakos EE, et al. Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J 2012;21:247-55. [Crossref] [PubMed]
- Hott JS, Deshmukh VR, Klopfenstein JD, et al. Intraoperative Iso-C C-arm navigation in craniospinal surgery: the first 60 cases. Neurosurgery 2004;54:1131-6; discussion 1136-7. [Crossref] [PubMed]
- Ishikawa Y, Kanemura T, Yoshida G, et al. Clinical accuracy of three-dimensional fluoroscopy-based computer-assisted cervical pedicle screw placement: a retrospective comparative study of conventional versus computer-assisted cervical pedicle screw placement. J Neurosurg Spine 2010;13:606-11. [Crossref] [PubMed]
- Ishikawa Y, Kanemura T, Yoshida G, et al. Intraoperative, full-rotation, three-dimensional image (O-arm)-based navigation system for cervical pedicle screw insertion. J Neurosurg Spine 2011;15:472-8. [Crossref] [PubMed]
- Ito Y, Sugimoto Y, Tomioka M, et al. Clinical accuracy of 3D fluoroscopy-assisted cervical pedicle screw insertion. J Neurosurg Spine 2008;9:450-3. [Crossref] [PubMed]
- Laine T, Schlenzka D, Mäkitalo K, et al. Improved accuracy of pedicle screw insertion with computer-assisted surgery. A prospective clinical trial of 30 patients. Spine (Phila Pa 1976) 1997;22:1254-8. [Crossref] [PubMed]
- Lekovic GP, Potts EA, Karahalios DG, et al. A comparison of two techniques in image-guided thoracic pedicle screw placement: a retrospective study of 37 patients and 277 pedicle screws. J Neurosurg Spine 2007;7:393-8. [Crossref] [PubMed]
- Ravi B, Zahrai A, Rampersaud R. Clinical accuracy of computer-assisted two-dimensional fluoroscopy for the percutaneous placement of lumbosacral pedicle screws. Spine (Phila Pa 1976) 2011;36:84-91. [Crossref] [PubMed]
- Ryang YM, Villard J, Obermüller T, et al. Learning curve of 3D fluoroscopy image-guided pedicle screw placement in the thoracolumbar spine. Spine J 2015;15:467-76. [Crossref] [PubMed]
- Wagner R, Telfeian AE, Iprenburg M, et al. Transforaminal Endoscopic Solution to a Kyphoplasty Complication: Technical Note. World Neurosurg 2016;91:195-8. [Crossref] [PubMed]
- Telfeian AE. An awake, minimally-invasive, fully-endoscopic surgical technique for treating lumbar radiculopathy secondary to heterotopic foraminal bone formation after a minimally invasive transforaminal lumbar interbody fusion with BMP: technical note. J Spine Surg 2018;4:162-6. [Crossref] [PubMed]
- Telfeian AE. Transforaminal endoscopic solution to disk reherniation post-mini-TLIF: case report. Clin Neurol Neurosurg 2015;131:69-71. [Crossref] [PubMed]
- Wagner R, Telfeian AE, Krzok G, et al. Transforaminal Endoscopic Decompression for Displaced End Plate Fracture After Lateral Lumbar Interbody Fusion: Technical Note. World Neurosurg 2017;106:26-9. [Crossref] [PubMed]
- McGrath LB Jr, Madhavan K, Chieng LO, et al. Early experience with endoscopic revision of lumbar spinal fusions. Neurosurg Focus 2016;40:E10. [Crossref] [PubMed]
- Telfeian AE, Oyelese A, Fridley J, et al. Transforaminal Endoscopic Decompression for Foot Drop 12 Years After Lumbar Total Disk Replacement. World Neurosurg 2018;116:136-9. [Crossref] [PubMed]
- Wagner R, Iprenburg M, Telfeian AE. Transforaminal endoscopic decompression of a postoperative dislocated bone fragment after a 2-level lumbar total disc replacement: case report. Neurosurg Focus 2016;40:E8. [Crossref] [PubMed]


