Adult intradural intramedullary astrocytomas: a multicenter analysis
Introduction
Intramedullary astrocytomas represent a rare neoplasm constituting approximately 2–4% of all primary central nervous system (CNS) tumors (1). These neoplasms arise from star-shaped glial cells, or astrocytes, and are often associated with a poor prognosis. Due to the very-low incidence of these tumors, there is a paucity of data on prognostic factors and the outcomes following the treatment of patients with these tumors. Additionally, the diagnosis and treatment of these neoplasm often presents a challenge given their enigmatic presentations as back pain, extremity weakness, paresthesia, and bowel and bladder dysfunction (2-4). The distinct location and extent of tumor spread often dictates the presence or absence of neurological findings.
Several studies have been undertaken at better understand the variables and outcomes associated with these neoplasms. Due to the difficulties surrounding its clinical diagnosis, uncertainty regarding optimal surgical management and the ineffectiveness of non-surgical treatment, most reviews of intramedullary astrocytomas are small retrospective analyses, case studies, or based upon data spanning changes in treatment paradigms and ability (5-11). Thus, there continues to be a need for recent clinical studies incorporating larger patient cohorts in order to more adequately assess the epidemiological and survival risk factors associated with these neoplasm in the hope of optimizing outcomes and treatment. To this end, in this population-based retrospective study, we explore various survival risk factors in adult patients with intramedullary astrocytomas.
Methods
Study design and selection of patient cohort
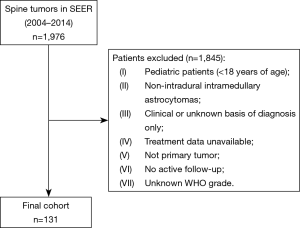
This was a population-based retrospective analysis of survival of adult patients (ages greater than 18 years of age) with intramedullary astrocytomas. We utilized the Surveillance, Epidemiology, and End Results (SEER) database, which is a prospectively collected cancer registry actively maintained by the National Cancer Institute (NCI). This analysis utilized the most recent publication of the “Incidence—SEER 18 Registries Research Data and Hurricane Katrina Impacted Louisiana Cases” from the 2016 submission, which contains cases from the years 1973 to 2014, however, only patients diagnosed between 2004 and 2014 were included in this study, as these were the only years during which treatment data was reported in the database. All adult patients with primary intramedullary astrocytomas classified by International Classification of Diseases for Oncology, Third Edition (ICD-O-3) were included in this study. Patients reported with a non-primary tumor, lacking active follow-up, having their tumor diagnosed on autopsy, a diagnosis confirmed by means of a non-histological modality (such as radiography, direct visualization, clinical diagnosis, or unknown modality), or an unknown WHO tumor grade were excluded from this study (Figure 1).
Variables
Variables of interests were chosen using the SEER database. The outcome variable used in this study was survival status. All variables were analyzed with respect to survival status. Exposure variable include age, sex, race, tumor size, tumor grade, tumor extension, radiation treatment and sequence, chemotherapy, and surgery. Age, sex, and race were self-reported by each patient within the SEER database. Tumor size was measured in millimeters and was based on the size of the neoplasm at the time of diagnosis. Tumor grade was determined based on the WHO Classification of tumors of the CNS and included pathological examination of the neoplastic tissue. Radiation included a combination of external beam radiotherapy and radiotherapy not otherwise specified.
Statistical analysis
Categorical variables were analyzed using the Fisher exact test or chi-square test was used, as respectively appropriate. The Student’s t-test was utilized for the comparison of continuous variables. Survival time was determined as the interval described in months between diagnosis and death or if the patient was alive, last follow-up reported in SEER. Kaplan-Meier curves were generated for visual characterization of survival by various variables. An accelerated failure time (AFT) regression was utilized to adjust for confounding, and the resulting coefficients in the model were converted to hazard ratios for interpretation. Survival rates were calculated at 1-, 3-, 5-, and 10-year intervals. All P values were reported as two-sided, with statistical significance being defined as P values <0.05. Statistical analysis was performed using R statistical software (version 3.4.2, 2017).
Results
Baseline demographics
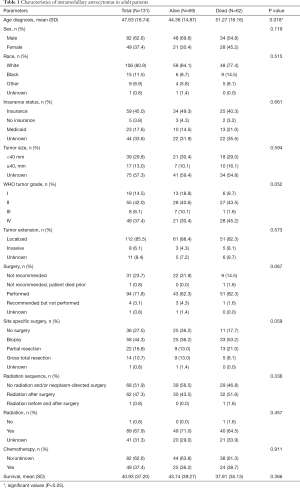
Between the years of 2004 and 2014, there were 131 patients recorded in the SEER database with histologically identifiable intramedullary astrocytomas neoplasms whose age was greater than 18 and whose survival time was documented (Figure 1). Baseline descriptive characteristics of the complete cohort stratified by survival status were compared (Table 1). The average age of this population at diagnosis (± SD) was 47.63±16.74 years, and 49 (37.4%) were female. The majority of the population was Caucasian (n=106, 80.9%), while 15 (11.5%) were African American, and 9 (6.9%) had been categorized as other. Fifty-nine (45.0%) patients had insurance, 23 (17.6%) were on Medicaid, and 5 (3.8%) had no insurance. By the end of data compilation, 69 patients were alive and 62 were deceased.
Full table
The characteristics of these intramedullary astrocytomas included, 39 (29.8%) that were smaller than 40 mm in maximum diameter and 17 (13.0%) that were 40 mm or larger in size. The majority of the intramedullary astrocytomas remained localized and did not invade surrounding structures (n=112, 85.5%), while 8 (6.1%) were invasive. Stratified by WHO classification, 19 (14.5%) of the tumors were grade I, 55 (42.0%) were grade II, 8 (6.1%) were grade III, and 49 (37.4%) were grade IV. WHO grades I and II were also combined to represent low-grade tumors and made up 62.6% of the tumor population with high-grade tumors (WHO grades III and IV) comprising 37.4%. High-grade tumors showed a worse prognosis with survival rates of 1, 5, and 10 years (73.5%, 32.5%, and 0.00% respectively) compared to low-grade tumors (93.0%, 64.8%, and 34.2% respectively). The predominance of these patients had surgery in order to decrease tumor burden (n=94, 71.8%), with 22 (16.8%) patients undergoing partial resections and 14 (10.7%) having gross-total resections. A minority of these patients, 36 (27.5%) did not undergo any surgery. Fifty-eight (44.3%) patients had undergone a biopsy. Additionally, a majority of these patients underwent radiation therapy (n=89, 67.9%), while only 49 (37.4%) had undergone chemotherapy.
When discriminating by survival status, only age at diagnosis was significantly different between the two survival status groups, alive and dead. Sex, race, insurance status, tumor size, WHO grade, tumor extension, surgery, radiation sequence, chemotherapy, and mean survival were not significantly different between the two groups.
Survival analysis
Overall survival at 1 year, 3 years, 5 years and 10 years, were found to be 85.5%, 71.0%, 64.1%, and 55.0% respectively. Kaplan-Meier log-rank analysis demonstrated age 80 or older at diagnosis (Figure 2A), tumor grade (Figure 2B), and chemotherapy treatment (Figure 2C) were possibly associated with worse overall survival. While race (Figure 2D), tumor extension and size (Figure 2E,F), surgery (Figure 3), radiation treatment and sequence (Figure 4A,B) did not reach statistical significance. This univariate and unadjusted analysis also did not control for any confounding variables that might have been present.
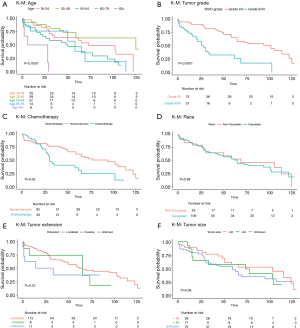
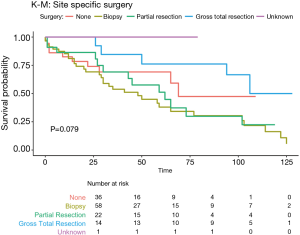
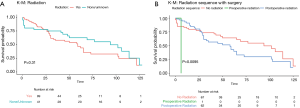
Sex, race, tumor size, low-grade tumors, treatment with radiation, and radiation-surgery sequence had no statistically significant associations with survival in either uni- or multivariate analysis (Table 1).
On univariate AFT regression, a 1-year increase in age of diagnosis, tumor grade IV classification, treatment with chemotherapy, and biopsy alone each had statistically significant negative associations with survival, while gross total resection had a statistically significant positive association with survival (P<0.05, Table 2). After adjusting for the confounding effects of each variable, our multivariate AFT survival analysis showed that a 1-year increase in age of diagnosis [hazard ratio (HR) 1.52, 95% CI: 1.17–1.99, P=0.001], WHO grade IV classification (HR 8.85, 95% CI: 2.83–27.69, P<0.001), tumor invasiveness (HR 2.94, 95% CI: 1.00–8.64, P=0.047), tumor biopsy alone (HR 6.26, 95% CI: 1.41–27.69, P=0.016), and sub-total resection (HR 5.80, 95% CI: 1.20–28.03, P=0.029) were each associated with worse overall survival (Table 2).
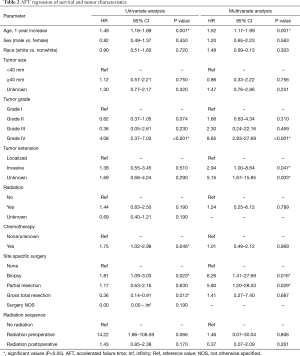
Full table
In univariate analysis, both the positive effect on survival of gross total resection and the negative effect on survival in patients receiving chemotherapy were statistically significant but that significance was lost in multivariate regression (when all factors were included). Both invasive tumor extension and partial surgical resection of tumor were not statistically significant factors in univariate analysis but both gained a significant negative impact on survival in multivariate analysis (Table 2).
Discussion
Intramedullary astrocytomas represent a rare neoplasm constituting approximately 2–4% of all primary CNS tumors (4). There is a paucity of studies assessing the long-term survival outcomes of adult patients with these neoplasms. To this end, 131 adult patients from the SEER database were evaluated with regard to a number of factors. Overall survival of these patients at 1 year, 3 years, 5 years and 10 years, were found to be 85.5%, 71.0%, 64.1%, and 55.0% respectively. Age at diagnosis (P=0.001), tumors of grade IV (P=0.000), invasive tumor extension (P=0.047), biopsy alone (P=0.016) and sub-total resection (P=0.029) were each associated with decreased survival and worse long-term prognosis. Sex, race, tumor size, radiation and chemotherapy were not found to impact the survival of patients.
The overall survival seen in this study is consistent with what previous literature has reported. A case series of 30 patients with spinal cord astrocytomas conducted by Nakamura et al. demonstrated overall survival rates of 68% at 5 years and 36% at 10 years. Significant predictors of survival rates included low-grade gliomas compared to high-grade gliomas (P=0.0011) and location of the neoplasm, with thoracic spinal cord astrocytomas having improved survival compared to those located in the cervical region (P=0.0251) (12). Robinson et al. also demonstrated higher overall survival in patients with low-grade astrocytomas of the spinal cord. This cohort consisted of 14 patients with pathology confirmed low-grade gliomas and were treated with either biopsy (n=7), subtotal resection (n=6), or gross total resection (n=1). Ten of these patients then underwent adjuvant radiotherapy (median total dose 50 Gy in 28 fractions). Overall survival of this cohort at 5, 10, and 20 years was 100%, 75%, and 60%, respectively. While our overall survival rates are very good with regard to this aggressive neoplasm, our cohort consisted of more low-grade gliomas compared to high-grade ones (62.6% vs. 37.4%) as well as many patients receiving some method of intervention whether it was radiation (48.1%) or surgery (subtotal or gross-total resection =27.5%) which can help explain our improved results compared to other studies that might have shown decreased survival rates (13).
Spinal cord neoplasms are relatively uncommon, accounting for approximately 1,700–2,700 of the more than 17,000 newly diagnosed primary CNS lesions in the United States each year (6,14). Tumors of the spinal cord are typically classified into three categories, extradural, intradural extramedullary, and intramedullary depending on the location within the CNS (6). The treatment for these types of neoplasm is often surgical with gross total resection being the standard of care. However, caution must be taken when operating on these types of tumors as damage to cardiovascular, pulmonary, motor, and neurological functions can lead to lasting complications (6). In this study, both tumor biopsy alone and sub-total resection were associated with significantly worse overall survival compared to those who received gross total resection at 1 year, 5 years and 10 years after surgery. While surgical resection still remains the best method of treatment for patients with these types of neoplasms, some studies have shown little to no effect on survival following surgery, and in some cases, surgery was associated with worse post-operative outcomes (3,15-17). In a cohort study of 202 patients with intramedullary spinal cord tumors conducted by Raco et al. (17), 61% of the patients with grade III and IV tumors actually had worst post-operative functional ability compared to those patients who did not undergo any surgery. Another study by Garcés-Ambrossi et al. (16) also found worse functional and survival outcomes in a retrospective analysis of 101 patients with intramedullary spinal cord tumors. Specifically, patients with astrocytomas showed no improvement in progression-free survival while other CNS neoplasms (i.e., ependymomas and hemangioblastoma) responded more favorably to surgical resection, possibly demonstrating that the histology of the tumor has a role on long-term outcomes.
Astrocytomas represent a subset of CNS neoplasm that can vary greatly in their site of growth and outcomes. The grading of these tumors is achieved using the WHO Classification of the Central Nervous System, with grades ranging from I to IV depending on the various histological appearances and molecular features of the tumor (18). Typically, higher graded neoplasms (III and IV) demonstrate a more aggressive nature with greater mitotic activity, poorly differentiated features, and potential to invade beyond the basement membrane of the organ, ultimately leading to worse prognosis (18). Our analysis showed that low-grade tumors made up 62.6% of the tumor population with high-grade tumors representing the remaining 37.4%. High-grade tumors (specifically grade IV) showed a worse prognosis with survival rates of 69.3%, 25.6%, and 0.00% at 1, 5, and 10 years respectively. This is consistent with other studies that have shown higher grade tumors leading to worse survival outcomes and greater long-term adverse effects (15,19-21). In a retrospective analysis conducted by Abdel-Wahab et al. that included 57 patients with a diagnosis of astrocytoma, overall survival of their cohort at 5, 10, and 15 years was 59%, 53%, and 32% respectively. Their cohort consisted of four patients with high-grade gliomas (WHO III/IV), 20 low-grade gliomas (WHO I/II), and 33 patients with unknown grade astrocytomas. However, their analysis showed a statistically significant difference on overall survival based on the grade of the neoplasm (P<0.01) (22). Two separate review articles by Houten et al. (20) and Benes et al. (15) also demonstrated worse survival outcomes for patients with high-grade intramedullary astrocytomas compared to those with low-grade tumors. The cohorts reviewed by Houten et al. had an overall median survival time of 6 months in adult populations even with advanced surgery and treatment. The review conducted by Benes et al. (15) consisted of 35 studies of which, 14 looked at histological grade. All but one study showed a significant difference between low-grade and high-grade intramedullary astrocytomas with low-grade gliomas showing improved survival. The extremely poor outcomes and rapid progression of these tumors makes them difficult to treat and manage for long-term success.
The extension of a tumor into a neighboring or distant location of the body can profoundly impact the prognosis of the patient. In our cohort, patients had lower survival rates when their tumors showed invasive extension into adjacent structures (75.0%, 75.0%, 18.8%) compared to those whose tumors remained localized (87.6%, 52.6%, 27.0%) at 1, 5, and 10 years respectively. While tumor extension has not been extensively looked at in terms of long-term survival or function, some studies have shown similar relationships between decreased survival and function with increasingly invasive extension of the tumor beyond the site of origin (5,8,9). A study conducted by Ardeshiri et al. (5) shows that 22 patients with intramedullary spinal cord astrocytomas had significantly worse outcomes if the tumor involved more than three spinal cord segments. Surgical resection of these invasive tumors also leads to deteriorating outcomes compared to their more localized counterparts with those restricted to the cervical region showing the best prognosis. Another study of 46 patients with intramedullary spinal cord tumors by Ebner et al. (8) also demonstrated worse outcomes for patients whose tumors extended beyond three spinal cord segments. McCormick and Klekamp-Samii scores were assessed and showed that patients with greater longitudinal extension had worse neurological status pre-operatively, immediately post-operatively, and at 3-month follow-up.
While surgical intervention continues to be the mainstay treatment for intramedullary tumors, advancements in radiation and chemotherapy have expanded the therapy options for clinicians and offer patients an alternative choice to surgery. However, there is still some level of risk that must be taken into account with these treatments as factors such as optimal dosing and tumor proximity to intrinsic structures play vital roles in the effectiveness of their usage. Radiation (either stereotactic radiosurgery or external beam radiation) as a solo and adjuvant therapy continues to be explored as a viable treatment option for patients with the literature highlighting factors such as tumor location and histology as important components to its efficacy (10,11,23). The present study did not find any relationship between the administration of radiation or the sequence of surgery and radiation administration with improved survival outcomes for patients. A study conducted by Minehan et al. (10) of 79 patients who underwent surgery for spinal cord astrocytomas followed by radiation or no radiation showed that the greatest impact on survival was based on the histology of the neoplasm. Patients who were diagnosed with diffuse fibrillary astrocytomas responded better to radiation treatment following surgery compared to those diagnosed with pilocytic astrocytomas suggesting variations in the origin and molecular architecture of the neoplasm may play a role in their response to radiation treatment. Another study by Guidetti et al. (23) also demonstrated the importance of location and histology on the use of radiation therapy. Of the 129 intramedullary gliomas used in the study, 53 of them were astrocytomas. These patients demonstrated recurrence rates of 50% after an average of 4–5 years with inferior long-term functional results and survival compared to those receiving radiation and diagnosed with a different CNS neoplasm (i.e., ependymomas).
With the increasing interest in chemotherapy as an adjuvant or novel approach in the treatment of CNS neoplasms, there has been a resurgence in identifying subsets of patients who respond inadequately or are poor candidates for surgical resection and/or radiation therapy (15,17). The research into the efficacy of chemotherapy in the treatment intramedullary tumors has provided mixed results in the literature, with a study by Doireau et al. (7) showing evidence of its effectiveness in surgery-intolerant pediatric patients, while others have shown no effect in the adult population (15,24,25). Currently, platin-based chemotherapy agents have been used, but a recent study by Chaskis et al. has shown the effectiveness of temozolomide in treating a limited sample of five patients with WHO grade II intramedullary astrocytomas. All five patients were still alive at 10-year follow-up with stable disease burden following a 12-cycle regimen of temozolomide after surgical debulking (26). Our cohort of adult patients did not show any relationship between the use of chemotherapy in the treatment of intramedullary astrocytomas and long-term survival. These findings are consistent with other studies which have shown little improvement in survival and functional outcomes with the use chemotherapy. A study by Santi et al. (25) showed no significant survival benefit in patients receiving chemotherapy with radiation therapy compared to those receiving radiation only. An independent study by McGirt et al. also demonstrated no survival benefit in patients receiving post-operative adjuvant chemotherapy (24). In their cohort, 88% of patients diagnosed with glioblastoma multiforme showed a significant decrease in neurological function and a median survival of 9 months following chemotherapy while a separate group of 48% patients diagnosed with anaplastic astrocytomas demonstrated no effect. Chemotherapy remains a potentially valuable treatment option, however, more research needs to be done to determine which individuals will benefit most from this particular therapy.
Age also seems to have a significant role in the prognosis of intramedullary astrocytoma patients with older individuals showing worse outcomes compared to their younger counterparts (18,27,28). The difference between the two groups can possibly be explained by a number of factors such as cytogenetic variations that can lead to more aggressive aberrations of the tumor with poor prognosis (2). Our cohort also shows similar trends as other studies with patients over 80 years old having a 5-year survival rate of 50.0%, with all other age groups having a survival rate above 75.0%. This trend continues to hold at 10 years post-operatively where patients over 80 years of age had a 0.0% survival rate which was far below that of every other age group.
Limitations
This analysis has limitations that have consequences regarding its interpretation, the majority of which are due to the restricted data complication within SEER. Though the database provides records from a large number of patients being treated at multiple centers, the data is limited in that it is not highly granular. In particular, while the utilization of radiation and chemotherapy in the management of these cases was reported, the doses of radiation and chemotherapeutic agents and their associated doses and regimens were not represented in the database. These data would have provided a better insight into the treatment and management of these patients, and a better understanding as to the tradeoffs of utilizing more aggressive treatment and weighing the reduction of tumor burden and recurrence with worsening morbidity and mortality in the CNS. Moreover, though the multivariate analysis included tumor grade and the extent of resection, it remains likely that radiation therapy was used with more frequency in higher grade neoplasms, subtotal resection cases, or no resection cases, each of which would be correlated with decreased survival time. Additionally, this database spans a period of 10 years and neurosurgical treatment for these types of neoplasms has likely progressed substantially over that period. Looking at the overall rates of survival in elderly patients independently may not adequately report for natural history/actuarial statistics. Finally, while randomized clinical trials are the gold standard for illustrating the role of various treatment types in adult patients with intramedullary spinal cord astrocytomas, such studies are difficult to perform due to the rarity of this disease. Although causality cannot be established due to the retrospective nature of this analysis, a strength of the study includes the increased cohort size relative to previously described studies. Additionally, the data was obtained from multiple healthcare centers across the United States, making this sample more representative of the general populace than the cohorts used in smaller, single-institution reports. This analysis reinforces many of the conclusions drawn from smaller data sets and emphasizes the need for prospective studies to provide additional insight into the survival risk factors in patients with these unique types of CNS neoplasms.
Conclusions
Our study of 131 adult patients with intramedullary spinal cord astrocytomas provides insight into factors that may influence survival. Higher WHO tumors, age at diagnosis, tumor invasiveness, and sub-total resection appear to have significant associations with worse prognoses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chamberlain MC, Tredway TL. Adult Primary Intradural Spinal Cord Tumors: A Review. Curr Neurol Neurosci Rep 2011;11:320-8. [Crossref] [PubMed]
- Cooper PR, Epstein F. Radical resection of intramedullary spinal cord tumors in adults. J Neurosurg 1985;63:492-9. [Crossref] [PubMed]
- Innocenzi G, Salvati M, Cervoni L, et al. Prognostic factors in intramedullary astrocytomas. Clin Neurol Neurosurg 1997;99:1-5. [Crossref] [PubMed]
- McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008;63:700-7; author reply 707-8. [Crossref] [PubMed]
- Ardeshiri A, Chen B, Hütter BO, et al. Intramedullary spinal cord astrocytomas: the influence of localization and tumor extension on resectability and functional outcome. Acta Neurochir (Wien) 2013;155:1203-7. [Crossref] [PubMed]
- Cooper PR. Outcome after operative treatment of intramedullary spinal cord tumors in adults: intermediate and long-term results in 51 patients. Neurosurgery 1989;25:855-9. [Crossref] [PubMed]
- Doireau V, Grill J, Zerah M, et al. Chemotherapy for unresectable and recurrent intramedullary glial tumours in children. Br J Cancer 1999;81:835-40. [Crossref] [PubMed]
- Ebner FH, Roser F, Falk M, et al. Management of intramedullary spinal cord lesions: interdependence of the longitudinal extension of the lesion and the functional outcome. Eur Spine J 2010;19:665-9. [Crossref] [PubMed]
- Kim MS, Chung CK, Choe G, et al. Intramedullary spinal cord astrocytoma in adults: postoperative outcome. J Neurooncol 2001;52:85-94. [Crossref] [PubMed]
- Minehan KJ, Shaw EG, Scheithauer BW, et al. Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg 1995;83:590-5. [Crossref] [PubMed]
- Paulino AC, Wen BC, Buatti JM, et al. Intracranial ependymomas: an analysis of prognostic factors and patterns of failure. Am J Clin Oncol 2002;25:117-22. [Crossref] [PubMed]
- Nakamura M, Chiba K, Ishii K, et al. Surgical outcomes of spinal cord astrocytomas. Spinal Cord 2006;44:740-5. [Crossref] [PubMed]
- Robinson CG, Prayson RA, Hahn JF, et al. Long-term survival and functional status of patients with low-grade astrocytoma of spinal cord. Int J Radiat Oncol Biol Phys 2005;63:91-100. [Crossref] [PubMed]
- Jung J, Choi W, Ahn SD, et al. Postoperative radiotherapy for ependymoma. Radiat Oncol J 2012;30:158-64. [Crossref] [PubMed]
- Benes V, Barsa P, Benes V, et al. Prognostic factors in intramedullary astrocytomas: a literature review. Eur Spine J 2009;18:1397-422. [Crossref] [PubMed]
- Garcés-Ambrossi GL, McGirt MJ, Mehta VA, et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine 2009;11:591-9. [Crossref] [PubMed]
- Raco A, Esposito V, Lenzi J, et al. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 2005;56:972-81; discussion 972-81. [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Ho VK, Reijneveld JC, Enting RH, et al. Changing incidence and improved survival of gliomas. Eur J Cancer 2014;50:2309-18. [Crossref] [PubMed]
- Houten JK, Cooper PR. Spinal cord astrocytomas: presentation, management and outcome. J Neurooncol 2000;47:219-24. [Crossref] [PubMed]
- Selvapandian S, Rajshekhar V, Chandy MJ. Brainstem glioma: comparative study of clinico-radiological presentation, pathology and outcome in children and adults. Acta Neurochir (Wien) 1999;141:721-6; discussion 726-7. [Crossref] [PubMed]
- Abdel-Wahab M, Etuk B, Palermo J, et al. Spinal cord gliomas: A multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys 2006;64:1060-71. [Crossref] [PubMed]
- Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg 1981;54:323-30. [Crossref] [PubMed]
- McGirt MJ, Goldstein IM, Chaichana KL, et al. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery 2008;63:55-60; discussion 60-1. [Crossref] [PubMed]
- Santi M, Mena H, Wong K, et al. Spinal cord malignant astrocytomas. Clinicopathologic features in 36 cases. Cancer 2003;98:554-61. [Crossref] [PubMed]
- Chaskis E, Minichini V, Luce S, et al. Contribution of temozolomide chemotherapy for intramedullary grade II spinal cord astrocytomas in adults: Our experience. Neurochirurgie 2017;63:297-301. [Crossref] [PubMed]
- Hirose Y, Aldape K, Bollen A, et al. Chromosomal Abnormalities Subdivide Ependymal Tumors into Clinically Relevant Groups. Am J Pathol 2001;158:1137-43. [Crossref] [PubMed]
- Lee HK, Chang EL, Fuller GN, et al. The prognostic value of neurologic function in astrocytic spinal cord glioma. Neuro Oncol 2003;5:208-13. [Crossref] [PubMed]