Multi-stage surgery for a multiple-level spondylodiscitis caused by multidrug-resistant Mycobacterium avium complex
Introduction
Mycobacterium avium complex (MAC) consists of at least two mycobacterial species, M. avium and M. intracellulare (1). MAC organisms are commonly in many environmental sites that can be isolated from soil, water, house dust, and animals (2).
MAC-associated extra-pulmonary infections, for example osteomyelitis, are relatively rare (3). Infection caused by MAC has been reported in distal femur, proximal tibia, calcaneus, ribs, ileum, wrist, knee, ankle and shoulder joints (4). Spinal infections are rare and only 70 cases of vertebral osteomyelitis caused by non-tuberculous mycobacteria have been reported of which 38 were caused by MAC (5,6).
Here, we report a rare case of antibiotic-resistant multi-level spondylodiscitis due to MAC that was treated successfully with multi-stage surgical treatment.
Case presentation
A 71-year-old female with a history of Sjogren’s Syndrome, osteoporosis, long-term steroid treatment and an infiltrative ductal carcinoma of the left breast treated with surgery in 2007, radiotherapy and subsequent chemotherapy, was diagnosed with spondylodiscitis due to an infection caused by MAC in June 2014.
Initially treated by antibiotic therapy (amikacin, clarithromycin, rifampicin and ethambutol). Two months after starting the treatment, the patient presented a cauda equina syndrome due to epidural compression in L5–S1 which required the execution of two separate decompressive L5 laminectomies. In the following months, the patient suffered severe hepatotoxicity and neuropsychiatric toxicity due to antibiotherapy, and clinically presented an increase in pain with inability to walk and sit.
In September 2015, the patient was referred to our institution for the treatment of her spondylodiscitis by hyperbaric therapy.
Among the tests carried out in our center, the anatomopathological and microbiological study of the bone marrow did not show signs of neoplastic proliferation. The cultures and the parasitological examination also were negative. The protein electrophoresis study showed no monoclonal peak. Blood tests showed a raised erythrocyte sedimentation rate of 52 mm/h and a C-reactive protein level of 0.77 mg/dL with a normal white cell count. The white cell differential counts were within normal ranges. The bone alkaline phosphatase level was normal. And a whole-body CT scan was negative.
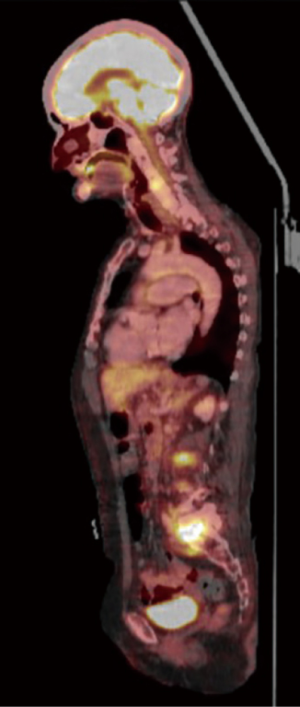
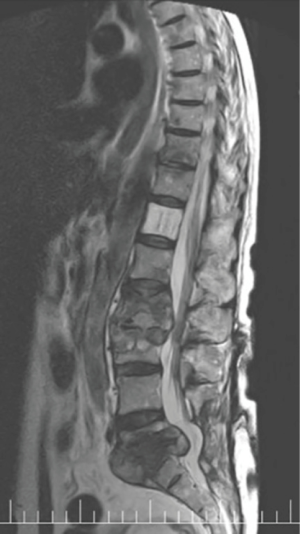
A SPECT scan (Figure 1) and an MRI of the whole spine were performed (Figure 2). The MRI study demonstrated diffuse multifocal infiltration of several lumbar and dorsal vertebral bodies by multiple hypointense deposits in T1 and hyperintense in TR sequences. In the vertebral bodies of L2 and L3 there was cortical rupture and prevertebral soft tissue mass. There was significant destruction of the intervertebral space and the vertebral plates at L5–S1, appreciating intense edema and osteitis of both vertebral bodies. A collection was observed in the disc space at L5–S1 compatible with discitis and abscessification.
A two-stage surgical treatment was chosen. The first aim was to stabilize the segments L2–L3 and L5–S1. In this way, we perform a posterior instrumentation with cement-augmented pedicle screws in L1, L4 and two bilateral iliac screws. We took advantage of this surgical intervention to take biopsies from prevertebral soft tissue mass in L2–L3 and from and the abscess at L5–S1. We did not decide to insert screws at L5 because the body of L5 was completely unstructured, and to have the possibility later, to perform a corpectomy of L5, which would be very complicated with the presence of pedicle screws in it. On the other hand, we decided to stop the instrumentation at L1 for three reasons: because the purpose of this first surgery was to solve the diagnostic problem and the lumbosacral mechanical incompetence, and because the thoracic spine showed no mechanical incompetence and we had radiological suspicions of possible infectious focus at T10–T11.
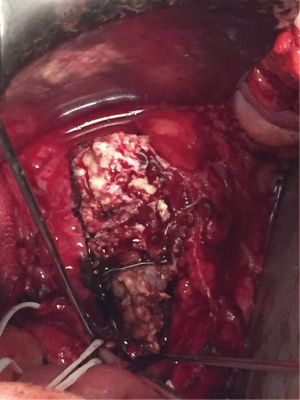
Three months later the patient underwent a second surgery with the aim of performing a resection of the infectious tissue and get an anterior lumbar interbody fusion. We planned an anterior approach for a complete vertebrectomy of L5 (Figure 3) and a partial corpectomy of S1, and the L4/5 intervertebral disc was then removed. A Pyramesh® titanium cage with Grafton™ demineralized bone matrix was then placed in the vertebral body cavity (Figure 4). We could not use a wider implant because the patient had a lower Aorto-Iliac bifurcation position (discretely distal to the L4–L5 disc) with fibrotic adhesions of the inflammatory mass and a very difficult mobilization, generating a very narrow approach to the implantation of the cage.
The immediate post-operative course was uneventful, and at 6 weeks after the second surgery the patient was able to perform progressive ambulation without assistance with excellent pain control.
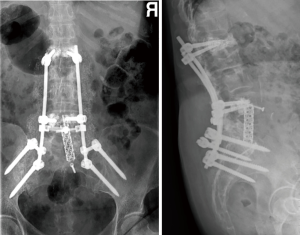
A year later, our patient started with pain and a positive sagittal imbalance as a consequence of a proximal junctional failure secondary to L1 vertebral fracture. At that time, we decided to perform a revision surgery of fusion extensions proximally, using percutaneous cemented screws in T10, T11 and T12.
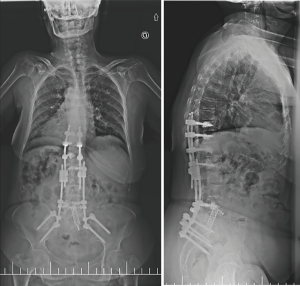
One week after the operation, the standing X-rays revealed a marked correction of the sagittal balance (Figure 5) and the patient was discharged from the hospital on the seventh postoperative day. There were no further complications.
The patient demonstrated a significant improvement in her symptoms with an overall satisfactory outcome and in the present moment she remains under follow-up in outpatient clinic by the spinal and infection diseases’ teams.
Discussion
Presumably, MAC osteomyelitis occurs after inhalation or ingestion of organisms followed by mycobacteriemia and secondary spread to bone. Alternatively, osteomyelitis may occur secondary to hematogenous spread, direct inoculation, and contiguous spread from infected pulmonary, gastrointestinal, or regional lymph nodes that erode into adjacent bony structures (4).
Infection caused by these bacteria is common in immunocompromised patients and the most frequent clinical expressions are pulmonary disease, cervical lymphadenitis, and disseminated disease in patients with acquired immune deficiency syndrome. However, MAC infection should be included in the differential diagnosis of severe bone pain in HIV-negative patients (7).
The clinical features are often indistinguishable from those of pyogenic osteomyelitis, particularly osteomyelitis due to Mycobacterium tuberculosis. Fever, weight loss, localized pain, lymphadenopathy and hepatosplenomegaly have been described as common symptoms in disseminated MAC infection (8,9).
Mycobacterial vertebral osteomyelitis has an indolent clinical course, although it can cause very severe sequelae including paraplegia due to spinal cord compression (10).
Another characteristic of mycobacterial spondylodiscitis is that because the spine manifests destructive changes in the advanced stages of these diseases, patients suffer severe back pain because of instability of the infected segment (11).
In MAC osteomyelitis, radiographic imaging shows non-specific findings. Radiographs may show rarefaction of the vertebral body, followed by disc space narrowing, involvement of adjacent vertebrae, and eventual destruction and collapse (10,12). Furthermore, magnetic resonance imaging mimic metastatic disease of the spine (7).
Differential diagnosis should include vertebral tuberculosis and tumor metastasis of the spine.
Skin and blood tests are not completely useful for diagnosis and differentiation from other infections, so the diagnosis of MAC infection is consequently established solely by isolation and identification of the microorganism from culture samples of biopsy material.
MAC is usually and is highly resistant to most anti-tuberculosis drugs (11,13,14), and frequently a combination of antibiotics should be used initially for patients suspected of having disseminated MAC infection (9,14).
Generally, indications for surgery in spinal osteomyelitis are neurologic deficit, abscess formation, uncontrolled infection or instability of the spine due to extensive bone destruction (5,9).
In cases of spondylitis in which conservative antibiotic therapy fails, surgical treatment has been recommended to correct and stabilize the unstable segment and to make a radical debridement of all necrotic tissues to treat spinal infection caused by Mycobacteria (15,16).
Two-stage surgical treatment (which consists of initial posterior instrumentation and secondary anterior debridement and interbody fusion) has been applied with excellent results in nine cases of spinal osteomyelitis due to Mycobacteria (10,11).
Combined anterior and posterior procedures are based on four principles according to Fukuta et al. (11). First, because shorter surgeries are associated with lower blood loss and less complications. Second, the use of posterior instrumentation in the first procedure offers the patient an attempt to gain stability and to encourage early ambulation. Third, because the immobilization of the affected segment of the spine could help in the suppression of spinal infection (17,18). Last, because the posterior column of the spine rarely the focus of the initial infection (19).
In our case, we decided to perform a two-stage surgical treatment. A first surgical procedure in order to perform posterior stabilization of the lumbosacral junction and achieve a definitive and updated diagnosis. It should be remembered that with the tumor history and despite the diagnosis of spondylodiscitis, our patient had not presented any clinical improvement with antibiotic therapy. And a second surgical intervention by anterior approach to make a resection of the abscessed tissue and give mechanical support to the anterior column of the lumbosacral junction.
In the initial surgical planning, we had proposed a third surgery for debridement and stabilization of segment L2–L3 by XLIF approach, but in the subsequent checks by MRI we could verify that segment L2–L3 showed improvement thanks to the posterior fixation and the antibiotic treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Daley CL. Mycobacterium avium Complex Disease. Microbiol Spectr 2017;5. [Crossref] [PubMed]
- Nishiuchi Y, Iwamoto T, Maruyama F. Infection Sources of a Common Non-tuberculous Mycobacterial Pathogen, Mycobacterium avium Complex. Front Med (Lausanne) 2017;4:27. [Crossref] [PubMed]
- Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 2014;6:210-20. [PubMed]
- Wood BR, Buitrago MO, Patel S, et al. Mycobacterium avium Complex Osteomyelitis in Persons With Human Immunodeficiency Virus: Case Series and Literature Review. Open Forum Infect Dis 2015;2:ofv090. [Crossref] [PubMed]
- Kim CJ, Kim UJ, Kim HB, et al. Vertebral osteomyelitis caused by non-tuberculous mycobacteria: Predisposing conditions and clinical characteristics of six cases and a review of 63 cases in the literature. Infect Dis (Lond) 2016;48:509-16. [Crossref] [PubMed]
- Gerogianni I, Boutlas S, Karachalios T, et al. A M. avium complex spondylodiscitis in a middle-aged woman with diabetes. Respir Med Case Rep 2017;21:71-3. [Crossref] [PubMed]
- Wang CS, Feng SW, Huang LJ, et al. Atypical mycobacterial spondylitis as a challenging differential diagnosis to metastatic disease of the spine: a case report. Eur J Orthop Surg Traumatol 2013;23 Suppl 2:S135-9. [Crossref] [PubMed]
- Jones AR, Bartlett J, McCormack JG. Mycobacterium avium complex (MAC) osteomyelitis and septic arthritis in an immunocompetent host. J Infect 1995;30:59-62. [Crossref] [PubMed]
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong) 2008;16:359-63. [Crossref] [PubMed]
- Hirakawa A, Miyamoto K, Ohno Y, et al. Two-stage (posterior and anterior) surgical treatment of spinal osteomyelitis due to atypical mycobacteria and associated thoracolumbar kyphoscoliosis in a nonimmunocompromised patient. Spine (Phila Pa 1976) 2008;33:E221-4. [Crossref] [PubMed]
- Fukuta S, Miyamoto K, Masuda T, et al. Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine (Phila Pa 1976) 2003;28:E302-8. [Crossref] [PubMed]
- Pruitt TC, Hughes LO, Blasier RD, et al. Atypical mycobacterial vertebral osteomyelitis in a steroid-dependent adolescent. A case report. Spine (Phila Pa 1976) 1993;18:2553-5. [Crossref] [PubMed]
- Pirofsky JG, Huang CT, Waites KB. Spinal osteomyelitis due to Mycobacterium avium-intracellulare in an elderly man with steroid-induced osteoporosis. Spine (Phila Pa 1976) 1993;18:1926-9. [Crossref] [PubMed]
- Lee CW, Sung HD, Choi BM, et al. Mycobacterium avium arthritis with extra-articular abscess in a patient with mixed connective tissue disease. Korean J Intern Med 2003;18:119-21. [Crossref] [PubMed]
- Klöckner C, Valencia R. Sagittal alignment after anterior debridement and fusion with or without additional posterior instrumentation in the treatment of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976) 2003;28:1036-42. [Crossref] [PubMed]
- Bailey HL, Gabriel M, Hodgson AR, et al. Tuberculosis of the spine in children. Operative findings and results in one hundred consecutive patients treated by removal of the lesion and anterior grafting. J Bone Joint Surg Am 1972;54:1633-57. [Crossref] [PubMed]
- Weisz RD, Errico TJ. Spinal infections. Diagnosis and treatment. Bull Hosp Jt Dis 2000;59:40-6. [PubMed]
- Broner FA, Garland DE, Zigler JE. Spinal infections in the immunocompromised host. Orthop Clin North Am 1996;27:37-46. [PubMed]
- Arthornthurasook A, Chongpieboonpatana A. Spinal tuberculosis with posterior element involvement. Spine (Phila Pa 1976) 1990;15:191-4. [Crossref] [PubMed]