
Minimally invasive approach to the lumbosacral junction with a single position, 360° fusion
Introduction
Minimally invasive spine surgery (MISS) is based on several key principles which differentiate it from traditional open spine surgery. First, minimal disruption of the soft tissue envelope enables reduced blood loss, reduced incisional pain, and more rapid recovery for patients after surgical decompression and fusion. Innovative approaches, such as anterior, lateral antepsoas, and lateral transpsoas, allow for more effective disc removal and better biomechanical control over the anterior and middle columns of the spine while simultaneously protecting the soft tissue envelope of the posterior spine. Lastly, the use of technological advances in imaging science, implant design, biologic fusion adjuncts, and surgical approach improvements drive the field toward continual refinement and improvement, all with the goal of minimizing the impact of a major spine surgery on patients and their families.
The lumbosacral junction, located at the confluence of the L5 and S1 segments, is a vitally important segment for both short segment and long segment operations. For short (single segment) decompressions, the angulation of the sacral slope and L5/S1 disc space can be difficult to expose and the thin dorsal bony element can raise the risk of unintended durotomy and CSF leak. Furthermore, the angle required to have orthogonal exposure to the disc space can require an incision as cranial as the L3 or L4 level, something one can only confirm with pre-incision fluoroscopy or from many years of experience with MISS. When conducting a short segment fusion, rod passage can be difficult due to the slope of the sacrum and the medial-lateral difference of the pedicle screw starting points of L5 and S1 respectively. Furthermore, the short transverse process of the L5 vertebral body may not be sufficient bony surface area for a short segment fusion, one that is minimally exposed during a minimally invasive surgery (MIS) approach to the lumbosacral junction. Regardless of being the termination of a short segment or longer segment fusion operation, the biomechanical stresses at the lumbosacral junction can be as high as seven times greater than the body weight body weight, which causes strain on implants, whether interbody or posterior lateral in location and insertion point, and can also result in high rates of pseudarthrosis. As a result of the high biomechanical stresses, a pseudarthrosis at the lumbosacral junction is rarely asymptomatic in our patient population.
Because of these considerations, it has become our practice to offer our patients with pathology at the lumbosacral junction a combined anterior-posterior fusion operation utilizing an anterior, antepsoas approach to the disc space with interbody fusion followed by posterior instrumentation utilizing robotic assistance while still in the lateral position. Using a typical and illustrative case, we present the common signs and symptoms of a patient with lumbosacral degeneration, and our approach to a single position, 360° L5–S1 fusion.
Case presentation
The patient is a 39-year-old woman with a long-standing history of back and leg pain. She endorsed 90% back pain and 10% leg pain, of which both legs were affected equally. Due to her condition, she has missed over a year of work. She failed multiple rounds of injections and physical therapy, and had been evaluated by two other surgeons both of whom had recommended an open, posterior fusion. Her Oswestry Back Disability Index was 70. She had a normal neurological examination with 5/5 strength throughout. Her BMI was normal at 23. Imaging revealed spondylosis at L5/S1 with vacuum disc phenomenon, with central and bilateral neural foraminal stenosis. Relevant imaging is displayed in the figures below (Figure 1).
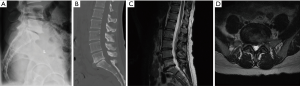
Anatomical, biomechanical, and logistical considerations for approach selection
Several unique anatomical features make the lumbosacral junction a difficult segment to manage in a minimally invasive fashion. Lateral access, such as a trans-psoas approach in more cranial segments of the lumbar spine, can be impossible to obtain due to the presence of the iliac crest. Direct anterior approaches, such as the anterior lumbar approach, can place critical neural and vascular structures at risk with long term consequences such as retrograde ejaculation, ureteral injury, abdominal hernia, or catastrophic vascular injury causing major blood loss (1). Posterior only approaches, either minimally invasive through tubular retractors or open, muscle splitting approaches, can result in disruption of the soft tissue envelope or destabilize the posterior tension band. Furthermore, posterior discectomy and interbody fusion can result in suboptimal discectomy and smaller graft implantation, greatly reducing the biomechanical strength of the construct and increased risk of subsidence, pseudarthrosis, and segmental kyphosis (2). Lastly, an understanding of the sacropelvic parameters and how they impact surgical corridors is of the upmost importance, as a patient with large pelvic incidence with minimal pelvic tilt can result in a large sacral slope which results in a L5/S1 disc space that is inaccessible from an anterior approach without a partial anterior L5 corpectomy.
Rationale for single position anterior and posterior approach
Anterior discectomy and interbody fusion are the preferred approach for the lumbosacral junction. First, many of the patient’s symptoms can be attributed to discogenic disease with collapse of the normal intervertebral space and sclerotic changes along the endplates of both L5 and S1. A thorough and complete discectomy can lessen and relieve this pain, and combined with the biomechanical advantage of engaging the entirety of the apophyseal ring of the endplates of L5 and S1 with the larger surface area for interbody fusion, a solid fusion of this segment can be curative in some patients. Furthermore, insertion of the graft anteriorly ensures optimal anterior column support which can be used to obtain segmental lordosis at this level commensurate with calculated ideal lumbar lordosis from the patient’s pelvic incidence. There is larger space through which to insert a much larger graft, which can in turn restore normal intervertebral height can provide indirect decompression of the neural foramina bilaterally and can better relieve radiculopathy than a similarly performed posterior interbody fusion. Lastly, the risk of iatrogenic nerve injury and CSF leak, as seen in posterior interbody approaches, is completely eliminated through an anterior approach.
Posterior fixation with a pedicle screw/rod construct is preferred for two reasons. First, the overall segmental stability of the anterior/posterior construct is greatly increased over a stand-alone anterior construct alone. Furthermore, the posterior instrumentation can be used to compress against the anteriorly positioned interbody device, resulting in appropriate restoration of segmental lordosis which is protective against adjacent segment degeneration. While the insertion of posterior instrumentation is preferred, the optimal positioning for anterior interbody grafts is typically supine, while posterior instrumentation is typically inserted with the patient prone on a Jackson table. The need for a position change, which involves moving the patient from the flat-topped operative table to a stretcher, then changing out of the bed to an open bottom Jackson style table, and then flipping the patient back prone can often take upwards of 45–60 minutes and be associated graft extrusion during flipping to loss of airway protection with inadvertent removal of the endotracheal tube. Through the use of a single position for both approaches, the operative time is greatly reduced by not only removing the need for repositioning but also by enabling the closure of one incision while the other is being exposed and instrumented at the same time. This reduction in OR time and elimination of the need for repositioning is most representative of the minimally invasive principles of reduced surgical invasiveness and reduced operative time.
Technique
Positioning
The patient is brought into the operating room and general anesthesia is induced. The patient is transferred onto the flat-topped Jackson table and placed into the right lateral decubitus position. The head rests on a foam donut and a pillow is placed between the legs. Typically, we bend the left leg to reduce the risk of a stretch injury during surgery but the right leg can stay straight. Then, two-inch silk tape is placed across the shoulder, the iliac crest, and the legs to firmly secure the patient to the table. This step is critical as the key to proper exposure and adequate radiographs is stable positioning in a true lateral position. Then, the fluoroscope is brought into the field and a radiopaque marker is used to plan the anterolateral incision for the antepsoas approach to the lumbosacral junction. Typically, this incision lies in line with the iliac crest and is inferolateral to the umbilicus. Once AP and lateral fluoroscopy confirm the incision, we make sure that the holder for the table mounted retractor is appropriately positioned on the table, and we also confirm that the connection for the robotic guidance system is positioned at the foot of the bed on the same side of the bed as the patient’s back is facing. Proper positioning of the patient and all necessary equipment is paramount to maintaining a brisk, efficient workflow and minimizing risk of complication at all time.
Equipment
We utilize several pieces of equipment and technology which greatly enhance the minimally invasive quality of this operative approach while also adding to the efficiency of the operative workflow. While not always necessary as the operation can be done using direct visualization, we prefer to utilize the operative microscope to enable a smaller incision with higher magnification and better illumination for the anterior portion of the operation. Furthermore, it enables better visualization for an assistant which can greatly speed up several key portions of the operation. Next, a table mounted retractor with expandable and angled retractor blades are a necessity for the operation, as the iliac arteries and veins must be retracted out of harm’s way to allow safe access to the L5/S1 disc space. The retractor system we commonly utilized also has integrated light sources which better illuminate the deep exposure through a smaller skin incision, which helps to reveal a small dog ear of vein or tissue that can be protected before damage occurs, which may not have happened if the periphery of the exposure was in the shadows of the microscope light. Lastly, we utilize a robotic navigation system for the posterior percutaneous instrumentation. More details regarding the robotic system will be discussed in the following section.
Operative technique and workflow
After positioning and confirmation of all necessary equipment, the patient is prepped and draped as per usual protocol. Then, the anterior portion is started first. The skin and deep dermal layer are opening, and the external and internal obliques are encountered. In our practice, we co-operate with a vascular surgeon who can help expedite the exposure but many other surgeons have performed their own approaches as well. We then dissect through the fat plane within the retroperitoneum down to the level of the iliac vessels. It is imperative that the preoperative MRI scan be analyzed to confirm the presence of a fat stripe beneath the vessels and anterior to the spine as well as an appropriately sized corridor between the vessels through which to gain access to the L5/S1 disc space. The presence of the fat stripe means that the vessels can be dissected and swept away from the prevertebral space without issue and the retractors can safely keep the vessels at bay during the discectomy. Additionally, the iliolumbar vein can lay directly across the disc space and must be identified and divided early to prevent inadvertent injury and large volume blood loss prior to any disc work being started.
Once the exposure has been completed and the vessels are protected, the discectomy can begin. The disc space is incised with a blade or the electrocautery device and a combination of Cobb elevators, disc shavers, and curettes can then be used to perform a thorough discectomy. The advantage of an anterolateral approach as opposed to a purely anterior approach is that more of the annulus can be released enabling the insertion of lordotic and hyperlordotic implants to aid in sagittal alignment restoration. In the case that there are prominent osteophytes, a high-speed drill or rongeurs can be used to take down these overgrowths and ensure proper sizing of the implant as well as placement of an anterior plate if needed. In our illustrative case, we elected to place and anterior plate primary as an anti-kick out mechanism. This can be inserted quite easily, with the path for the screws being largely in line with the angle of exposure from the anterolateral approach. If the screwdriver needs to be directed, it is usually more medially which involves dropping the hands towards the umbilicus, similar to direct anterior approach during an ALIF. In our experience, this is quite easy and provides great peace of mind that the graft will not displace anteriorly. The combined, single position approach as well as the use of the robotic navigation system afforded us the opportunity to do this in a streamlined, efficient workflow. To perform the registration for the robot, two radiographs are required to allow for the registration from the preoperative CT scan. The presence of a radiopaque plate, however, could possibly interfere with the acquisition of these X-rays and one of the top causes for registration failure with the robotic system is this issue. Thus, while the anterior procedure was still being performed, we could begin the posterior portion of the procedure while the patient was in the same position.
A small incision over the posterior superior iliac spine is performed and a Schantz pin is inserted into the PSIS for fixation of the robotic arm. Connection to the bed as well as the patient ensures a high level of security of the system as well as accuracy for implant insertion. Next, the robot is registered with the preoperative imaging and confirmation of the registration is performed on the system. One benefit of the software of this particular robotic system is that the registration is segmental and not volumetric, meaning changes in the disc height or removal of osteophytes during the anterior portion of the operation will not affect the use of a preoperative CT for registration. Then, screw trajectories and implant length can be planned and loaded into the system. The robotic arm can then be sent to each of the trajectories and a minimally invasive approach can be performed for each level. An inner and outer cannula are dropped through the guide on the actuator arm, and then a toothed cannula is gently impacted into place at the selected entry site. A thin flexible drill is then used to drill a pilot hole, and a small cannula and K-wire are dropped into the pedicle to secure the trajectory. The path is then tapped and the screw is inserted without the need to continuous fluoroscopic guidance. While this is being performed, the plate can be secured to the anterolateral aspect of the disc space and closure by the vascular surgeon can be commenced, as seen in the illustrative intraoperative photo (Figure 2).
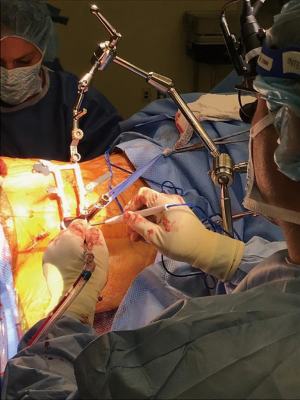
We prefer to use capped rods, which slide into the screw towers and can be dropped down into the screw heads and fully reduced using set screws. The benefit of this is that live fluoroscopy or serial fluoroscopic shots are not necessary to confirm that there is enough rod above and below the screw heads. Once all screws are final tightened, the towers can be removed and closure can proceed expediently and generally finishes at or about the same time as anterior closure. The drapes are then removed, and the patient can be turned back supine for reversal of general endotracheal anesthesia. We utilize continuous intraoperative neuromonitoring with somatosensory evoked potentials, transcranial motor evoked potentials, and free running electromyograms to ensure no neurologic deficit occurs during the procedure. We also typically use cell-saver, not because of the high blood loss, but in the event that there is a vascular injury blood can be recovered and transfused back to the patient. Our patients then go from the OR to the post anesthesia care unit, where they are typically seen by physical therapy on the same day.
Post-operative results
The patient tolerated the surgery well with only 100 cc of estimated blood loss. Total operative time for both portions of the procedure was 190 minutes. There were no blood transfusions. The patient was able to mobilize on post-operative day #0 with physical therapy was discharged home on post-operative day #3. Post-operative radiographs demonstrated ideal placement of pedicle screw implants and good restoration of intervertebral height and segmental lordosis with the interbody implant. At first follow up, there was a greater than 30-point reduction in her Oswestry Back Disability Index Score radiographs did not reveal any loss of correction or change in alignment (Figure 3).
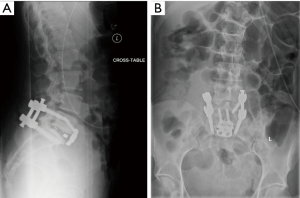
Future directions
With improvements in imaging techniques, navigated and robotic spine surgery could become important adjuncts in MISS. The use of these technologies could greatly reduce fluoroscopy time and increase efficiency in the operative workflow. Augmented reality could further enhance these techniques allowing for a real-time view of the spine within the soft tissue envelope, enabling safe and expedient access and instrumentation of the lumbosacral junction. This could integrate real-time navigation and robotic guidance and allow for better situational awareness during these cases. As our understanding of spinopelvic parameters and patient specific ideal alignment becomes better codified, we can utilize our pre-existing surgical techniques to better optimize personalized spine surgical techniques for each patient. Lastly, with improvements in fusion-adjunctive biologic agents less exposure of the spine can result in an equivalent fusion outcome as in open surgery with greatly reduced operative time, blood loss, and pain and morbidity to the patient. This multimodal approach to surgery of the lumbosacral junction could be crucial to not only MISS, but also in spinal deformity correction as well as the treatment of degenerative conditions.
Acknowledgments
The authors would like to acknowledge all the fellows, physician’s assistants, nurses, operating room and clinic staff of the Dan and Jane Och Spine Hospital at Columbia University Medical Center for the exceptional care provided for patients such as the one featured in this article.
Footnote
Conflicts of Interest: Dr. Lehman has the following conflicts of interest: Grant: PRORP (Department of Defense Peer Reviewed Orthopaedic Research Program)—paid directly to organization; Personal Fees/Financial Support: Dupuy Synthes Spine—Honoraria for Speakers Bureau, Travel paid for any speaking arrangements; Stryker—Honoraria for Speakers Bureau, Travel paid for any speaking arrangements; Medtronic—Consulting fees, Honoraria for Speakers Bureau, Travel paid for any speaking arrangements. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Shillingford JN, Laratta JL, Lombardi JM, et al. Complications following single-level interbody fusion procedures: an ACS-NSQIP study. J Spine Surg 2018;4:17-27. [Crossref] [PubMed]
- Hlubek RJ, Godzik J, Newcomb AGUS, et al. Iliac screws may not be necessary in long-segment constructs with L5-S1 anterior lumbar interbody fusion: cadaveric study of stability and instrumentation strain. Spine J 2019;19:942-50. [Crossref] [PubMed]


