
The use of robotics in minimally invasive spine surgery
Introduction
In 2019, autonomous technology is omnipresent, and the speed of technological innovation is ever increasing. Keeping pace, medical advances over the last century have changed the way people are both diagnosed with and treated for a disease process. Medicine has benefitted greatly from this revolution. Lister and Pasteur brought medicine out of the dark ages in the late 19th century. Robotics appears poised to revolutionize medicine in the 21st.
The field of spinal surgery has greatly benefited from these advancements, as constant improvements in diagnostic imaging, surgical magnification and illumination, and stereotaxis have allowed for safer, more efficacious operations. In the recent decades, a variety of minimally invasive surgical techniques have been introduced to minimize approach-related tissue trauma while optimizing peri-operative care and long-term functional outcomes (1,2). The goal of minimally invasive spine (MIS) surgery is to provide adequate decompression of neural elements and realign the spinal column where appropriate, while minimizing soft-tissue trauma. While few randomized controlled trials have been designed to compare MIS surgery to traditional, open surgery, there are many retrospective and prospective studies suggesting equal efficacy and safety between both approaches. Consistently, however, the MIS cohorts in these studies have less blood loss, less iatrogenic muscle injury, less peri-operative pain, and a decreased length of stay (3-7).
The history of MIS surgery dates back 80 years when Love published the first intralaminar approach to treat lumbar herniated discs. Later, in 1977, Yasargil and Caspar popularized the use of a microscope in operations to resect disc herniations (8). The first percutaneous transpedicular vertebroplasty and kyphoplasty were reported in 1984 and 2001 respectively (9,10). Arthroscopy for lumbar disc disease was first described by Forst and Hausma in 1983 and was further developed by Kambin years later. Kambin subsequently defined “Kambin’s triangle” as a safe posterolateral triangular working zone in 1996. Shortly thereafter, Foley and Smith reported their early experiences with tubular micro-endoscopic nerve root decompression in 1999 (11). With time, tubular and micro-endoscopic techniques gained popularity. Zucherman reported laparoscopic-assisted anterior lumbar interbody fusion (ALIF) surgeries in the 1990’s (12). Though the laparoscopic ALIF did not offer significant benefit over the open approach, it served as a gateway for other minimally invasive interbody fusion techniques in the lumbar spine. In 2002, percutaneous trans-pedicular screw-rod fixation was introduced by Foley as the Medtronic Sextant system, which allowed for multi-level instrumentation through two small paramedian incisions (13). Recently, image-guided, 3D, CT-based navigated systems have become more prevalent as these systems offer superior precision, safety, shorter operative time, and decreased radiation exposure for the operative team (14-17).
In the past decade, robotic surgical systems have been adopted in various surgical specialties to replace or complement traditional laparoscopic/endoscopic techniques (18-23). The term, “robot,” implies a machine capable of carrying out a complex series of actions automatically. A true spinal surgery robot has yet to be created. Rather, “cobots”—machines designed to interact and assist humans—have been created in an attempt to make spine surgery safer and more efficient.
Robotics in spinal surgery has evolved relatively quickly over the past decade. In 2004, the Mazor SpineAssist device was the first FDA approved robot used to guide the placement of pedicle screws. Newer robotic devices including the Mazor X (Medtronic and Mazor Robotics, Memphis, TN, USA), ExcelsiusGPS (Globus Medical, Inc., Audubon, PA, USA), and ROSA (Medtech Surgical, Inc., New York, NY, USA) have since been developed and FDA cleared for use in the spine (Figures 1 and 2). Each new device builds on the basic tenant that pedicle screw insertion should be safer, easier, and more efficient.
The utility of robotic technology in spinal surgery is currently under investigation and has yet to achieve widespread adoption. Multiple studies have reported early experiences with robot-assisted pedicle screw placement and the outcomes are promising (24-36). Nonetheless, the role of robotics in a routine spinal practice remains somewhat unclear.
Although there is ample evidence proving the benefits of MIS surgery in specific instances, there is a paucity of data on the benefits and or detriments of robotics in MIS surgery.
In this review we aim to present various applications of robotics in MIS surgery and potential future applications.
Robotic learning curve
As with learning any new skill or task, whether in or out of the operating room, there is a relatively steep learning curve with both MIS surgery and robotic spine surgery. There are numerous examples throughout the spine literature espousing the difficulty of becoming proficient at performing a minimally invasive transforaminal lumbar interbody fusion (MIS TLIF). Lee et al. documented significantly shorter operative times, less blood loss, and less fluoroscopy time after a surgeon’s 44th MIS TLIF (37). Another study claimed a 50% improvement in efficiency after a surgeon’s twelfth MIS TLIF and a 90% improvement by the 39th case (38).
Similar findings have been reported with robotic surgery. One study compared the proficiency of junior and senior residents at placing robotically-guided pedicle screws in the lumbar spine. While the result did not prove significant given the sample size, there was an obvious trend implying more experienced residents could place screws faster. In addition, the time to place a single screw decreased as the volume of screws increased (39).
Another study by Hu and Lieberman examined 150 consecutive, robot-assisted procedures done by the lead author to quantify the learning curve. The accuracy of screw placement seemed to increase up to and plateau after the thirtieth case. In this series, intraoperative screw revision decreased from 17% to 7% after the thirtieth case (40).
Schatlo et al. reviewed 1,265 robotically-assisted pedicles screws placed by 13 different surgeons. In this series the authors found that the number of misplaced screws drops significantly after the twentieth case. Based on their data, the authors suggest that surgeons new to robotics be proctored for their first 25 robotic procedures (41).
Radiation in MIS surgery
Free hand pedicle screw placement has a known inaccuracy rate that likely contributed to a rise in the use of fluoroscopic image guidance to assist in screw insertion. The advent of minimally invasive surgery only served to increase the use of fluoroscopy thus increasing surgeon radiation exposure even further (42). Newer technology, such as robotic guides and CT-guided 3D navigation systems, allow for the placement of pedicle screws with potential for significant reductions in radiation exposure for both the operative staff and surgeons.
Kantelhardt et al. reported a decrease in radiation time from 77 seconds/screw in conventional, open operations down to 43 seconds/screw and 27 seconds/screw in robot-guided open surgery and robot-guided percutaneous surgery, respectively (26). Another paper reported similar results with mean radiation time per screw in the conventional group being over double that in the robot-guided cohort (25).
Percutaneous pedicle screws
Percutaneous transpedicular instrumentation was first introduced in 1977 by Magerl to provide temporary external stabilization and fixation of spinal fractures (43). Since then, the technique has been described in several clinical studies that prove the efficacy, safety, and accuracy of this type of instrumentation (13,44-46). Studies examining the use of percutaneous screws demonstrate numerous, significant benefits when compared to open pedicle screw insertion, including a decrease in operative time, post-operative pain, blood loss, infection rate, and hospital length of stay (47-50). A retrospective analysis of percutaneous screw placement using fluoroscopy in vertebral levels T2 to S1 by Winder et al. revealed a 4.1% (25/614 screws) breach rate; with thoracic screws carrying a significantly higher rate of misplacement (51). The accuracy rates are better with 3D intra-operative navigation. A recent meta-analysis of 68 pertinent studies, including 3,442 patients, 60 cadavers and 43,305 pedicle screws, reported improvement in pedicle screw accuracy (defined as <2 mm breach) from 91.4% with fluoroscopy to 97.3% with CT-navigation (52).
There is a dearth of literature describing the use of robotics for the placement of percutaneous pedicle screws. Pechlivanis et al. published the first paper describing their experience with robotic placement of percutaneous pedicle screws. They found that the robotic system they used could place screws within two millimeters of the surgeon’s plan 91% of the time in the longitudinal plane and 98.3% in the axial plane (32). Another study compared the accuracy of robot-guided percutaneous screws to open robot-guided screws. As expected, there was no significant difference in accuracy between the open and percutaneously placed pedicle screws. However, they did note that following percutaneous screw placement, patients required less opioids, had a shorter length of stay, and had fewer adverse events than did comparable patients who underwent open robot-assisted instrumentation (26).
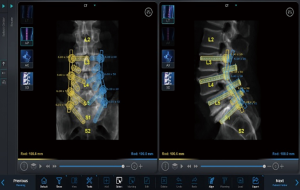
Hyun et al. completed a randomized, controlled trial evaluating minimally invasive, robotic-guided pedicle screw placement versus open, fluoroscopic-guided spinal instrumentation (25). They noted that the utilization of robot-guided percutaneous pedicle screws significantly reduced both radiation exposure as well as the length of stay compared to the control, fluoroscopy-guided instrumentation group. Importantly, they also found that there was a significantly increased distance between the proximal pedicle screw and the proximal facet in the robot-guided group. This might not acutely make a difference in patient reported outcomes, but over the long term, violation of the suprajacent facet leads to increased morbidity and a higher likelihood of adjacent segment disease. Preoperative planning of pedicle trajectory allows the surgeon to stay completely out of the facet (Figure 3). In addition, the workflow involved in placing robotic-guided screws does not allow for “stabbing” the facet capsule will trying to approximate a good fluoroscopic trajectory. This differs greatly when compared to the placement of fluoroscopy-guided screws with a Jamshidi needle. The facet capsule can be repeatedly violated when utilizing this technique. Patel et al. investigated the rate of facet violation in a cadaveric study and reported a 58% rate of facet capsule violation during fluoroscopy-assisted percutaneous pedicle screw insertion (53). Although likely unnoticed at the time of index surgery, this unperceived iatrogenic facet injury could possibly accelerate adjacent level facet degeneration and thus adjacent segment disease.
Robotics and bony decompression
Spine surgery is a physically demanding surgical subspecialty. Ergonomics have been widely studied in industry, the military, and in athletes. Researchers are now beginning to analyze ergonomics in the operating room (54,55). The incidence of neck and shoulder pain amongst surgeons have been reported to approach 40% (56). Moreover, with aging, a surgeon’s dexterity, precision, and stamina can become compromised. While current literature investigating possible correlation between aging and surgical outcome is conflicting, it is known that technical precision decreases with age (57-60). Spine surgeons rely on precision, dexterity, and control as they operate in the vicinity of critical neural elements and therefore are prone to age-related physical limitations. A review of other surgical subspecialties such as cardiac surgery, urology, and gynecology reveals that robotic surgery could potentially play a role in improving ergonomics and surgical outcomes (61-63). Ponnusamy et al. designed a porcine model to evaluate the role of the da Vinci Surgical Robot in performing posterior approaches to the spine including laminotomy, laminectomy, and dural closure (64). Unfortunately, at least in this study, the use of the robot required an open dissection of the spine, rendering some of the benefits of true MIS surgery moot. They did, however, show that the ergonomics provided by the robot decreased both mental and physical fatigue allowing the surgeon to operate for a longer duration with less fatigue. No other reports exist in the literature evaluating the use of robotics for spinal decompression signifying a marked void in need of future study.
Robot-assisted TLIF
TLIF is a common approach for treatment of degenerative spinal pathology introduced by Harms and Rolinger in 1982 to address some of the limitations associated with posterior lumbar interbody fusions (PLIF) (65). The main advantages of TLIF over a PLIF include a reduction in thecal sac and nerve root retraction, avoidance of midline scar tissue in revision cases, and circumferential fusion from a unilateral approach (66-68). In 2003, Foley et al. published their initial experience using tubular, minimally invasive retractor to perform a TLIF (69). Since then, multiple studies have reported reduced blood loss, less post-operative pain, decreased narcotic use, and shorter length of stay when using this minimally invasive approach as opposed to the traditional open approach (66,69,70). Chenin and Snyder reported their surgical experiences utilizing ROSA (Medtech Surgical, Inc., New York, NY, USA) spine robot and intraoperative O-arm CT navigation for minimally invasive TLIFs (71,72). This was not a controlled study, but rather a report of feasibility and integration of robotics into the TLIF workflow.
In another study, 96 patients with adult degenerative scoliosis underwent robot-assisted minimally invasive TLIF combined with the use of a gelatin sponge impregnated with a mixture of steroid, anesthetic, and a neuropathic drug. The MAZOR robot was used to instrument the spine in this series (73).
A retrospective study by Cui et al. investigated the efficacy of a robot-assisted MIS TLIF versus a traditional open approach for treatment of spondylolisthesis. The robot-assisted approach had less blood loss, shorter hospital length of stay, shorter time to first ambulation, less pain on post-operative day 3, and more precise pedicle screw placement. However, the robot-assisted cohort was subjected to longer operations and more radiation exposure (74). This method proved to be feasible and safe in another study and was associated with short length of stay and short-term analgesia effect though this study failed to include a control cohort for comparison (73).
There are unpublished reports of using a robot to guide a surgeon on the placement of pre-planned bony osteotomies for a precise facetectomy. This method allows for an exact detachment of the superior articular process just above the pedicle for optimal decompression and subsequent visualization during a TLIF (Figure 4).
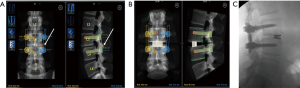
Robotically-guided facet decortication
Currently, in spinal surgery, robots serve simply as guides to aid in pedicle screw insertion. However, their applications in spinal surgery will continue to grow. Robotic spinal decompression has been studied in a porcine model, proving its efficacy (64). Spinal robots can provide precise guidance to any area of the spine that has been appropriately imaged and registered. In minimally invasive fusion operations, surgeons frequently attempt to decorticate the facets to provide another potential site of arthrodesis. Using the guidance of the robot, a surgeon can very easily access and decorticate a facet as seen in Figure 5. Utilizing the preoperative plan, the robotic arm simply swings in to position as if a pedicle screw were being inserted. However, rather than inserting a pedicle screw or tap, a large burr is inserted through the guide to facilitate facet decortication.
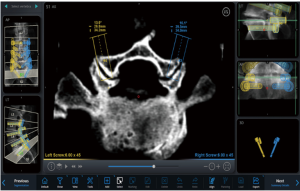
Robotics and navigation
In September 2018 Medtronic acquired Mazor Robotics. Soon thereafter, Medtronic announced they had developed a new robotics platform that combined their intraoperative CT-based spinal navigation system with the Mazor X robotic guidance platform. As seen in Figure 6, the combination of these technologies allows for real time instrument tracking while also still utilizing the pre-planned, robotically-guided pedicle screws. This, theoretically, adds another layer of safety to robotic pedicle screw instrumentation as any deviation from the planned trajectory would appear as a deviation from the plan on the navigation screen.
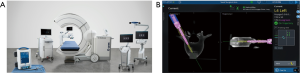
Robotics and anterior spinal surgery
The original report of ALIF dates to the early 1930’s (75). Since then multiple different anterior approaches to the lumbar spine have been entertained. In 1963, Harmon described the retroperitoneal approach to the anterior lumbar spine (76). The first attempt to apply MIS principles to the exposure of the anterior spine occur in the mid-1990s when Mathews and Zucherman first introduced the idea of the laparoscopic ALIF (12,77). Despite early promising results, a high complication rate, high rate of laparoscopic to open conversion, and a longer operative time resulted in this surgical technique falling out of favor. In 1999 Regan et al. conducted a large multi-center study comparing laparoscopic and open ALIFs and reported shorter hospital stay and reduced operative blood loss in laparoscopic cohort. While the complication rate was comparable between the two approaches, laparoscopic cohort had a higher rate of re-operation at 6 months and 10% of patients required conversion to the open approach surgery (78). Based on preliminary results, it was felt that the laparoscopic approach did not offer enough of an advantage to justify the long learning curve and technical difficulty associated with the procedure (75). In 1997 Mayer became the first to popularize the muscle sparing, minimally invasive approach utilized by majority of surgeons today (79). The minimally invasive technique is associated with less blood loss, shorter operative time, and improved clinical outcomes (80). In another large series, 686 patients underwent the mini-open approach described by Mayer with exposure time between 18.7 and 38.4 minutes depending on the disc level (81).
The da Vinci Surgical system is a telemanipulator robot that was FDA approved for laparoscopic surgery applications in 2000. The da Vinci system has been popularized and shown to be beneficial in numerous surgical specialties including gynecology, urology, and general surgery. This system is quite different from the current spinal robotic systems that act as pedicle insertion guides. The da Vinci system allows the robot to become an extension of the surgeon’s hands. It offers high definition, stereoscopic vision that can magnified up to 10× (82). Some surgeons have advocated for the use of the da Vinci Surgical systems robot to both expose the lumbar spine and help place instrumentation as an attempt to improve on the muscle sparing, retroperitoneal ALIF and the lackluster results of the laparoscopic approach.
Troude et al. performed an anatomical study proving the feasibility of using the da Vinci to perform either an anterior or oblique lumbar interbody fusion (83). Lee et al. retrospectively reviewed eleven cases in which they used the da Vinci robot to place anterior lumbar interbody grafts. All patients were noted to be fused on long term follow-up and there no major complications or conversions to open surgery. They did point out that mobilizing the vessels at L4–5 was quite difficult and time-consuming but could be done safely (82).
Beutler et al. developed a transperitoneal approach to L5–S1 utilizing the da Vinci system. After refining their technique on both a porcine and human cadaveric model, they presented a single case of a single level, standalone interbody fusion. They postulate that many of the advantages of robot-assisted abdominal surgery as reported in the general surgery, gynecologic, and urologic literature would apply to robotic ALIFs. Per Beutler, one would expect “decreased hospitalization time, lower morbidity, lower blood loss, [and] lower complication rates” (84).
In the literature, there have not been many attempts to adapt the da Vinci system to posterior spine surgery. Currently, it is only FDA approved for abdominal surgery.
Conclusions
Currently, the use of robots in spinal surgery is chiefly limited to the implantation of pedicle screws. However, many novel uses are likely on the horizon. With current technology, precise, robotically-guided facetectomies and facet decortication is possible. This implies a future in which surgeons utilize software to pre-plan minimally invasive decompressions and execute these plans with precision by utilizing a robotic arm for guidance. Technology does not currently allow for true, autonomous robotic spine surgery. Rather, ever-evolving cobots seem poised to make MIS surgery safer and more efficient.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kim DY, Lee SH, Chung SK, et al. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976) 2005;30:123-9. [Crossref] [PubMed]
- Jaikumar S, Kim DH, Kam AC. History of minimally invasive spine surgery. Neurosurgery 2002;51:S1-14. [Crossref] [PubMed]
- Wong AP, Smith ZA, Lall RR, et al. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg 2012;2012:325095. [Crossref] [PubMed]
- Fan SW, Hu ZJ, Fang XQ, et al. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg 2010;2:194-200. [Crossref] [PubMed]
- Kasis AG, Marshman LA, Krishna M, et al. Significantly improved outcomes with a less invasive posterior lumbar interbody fusion incorporating total facetectomy. Spine (Phila Pa 1976) 2009;34:572-7. [Crossref] [PubMed]
- Fessler RG, Khoo LT. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery 2002;51:S37-45. [Crossref] [PubMed]
- Ntilikina Y, Bahlau D, Garnon J, et al. Open versus percutaneous instrumentation in thoracolumbar fractures: magnetic resonance imaging comparison of paravertebral muscles after implant removal. J Neurosurg Spine 2017;27:235-41. [Crossref] [PubMed]
- Williams RW. Microlumbar discectomy: a conservative surgical approach to the virgin herniated lumbar disc. Spine (Phila Pa 1976) 1978;3:175-82. [Crossref] [PubMed]
- Belkoff SM, Jasper LE, Stevens SS. An ex vivo evaluation of an inflatable bone tamp used to reduce fractures within vertebral bodies under load. Spine (Phila Pa 1976) 2002;27:1640-3. [Crossref] [PubMed]
- Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987;33:166-8. [PubMed]
- Foley KT, Smith MM, Rampersaud YR. Microendoscopic approach to far-lateral lumbar disc herniation. Neurosurg Focus 1999;7:e5. [Crossref] [PubMed]
- Zucherman JF, Zdeblick TA, Bailey SA, et al. Instrumented laparoscopic spinal fusion. Preliminary Results. Spine (Phila Pa 1976) 1995;20:2029-34; discussion 2034-5. [Crossref] [PubMed]
- Foley KT, Gupta SK. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg 2002;97:7-12. [PubMed]
- Kalfas IH, Kormos DW, Murphy MA, et al. Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg 1995;83:641-7. [Crossref] [PubMed]
- Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976) 2007;32:E111-20. [Crossref] [PubMed]
- Murphy MA, McKenzie RL, Kormos DW, et al. Frameless stereotaxis for the insertion of lumbar pedicle screws. J Clin Neurosci 1994;1:257-60. [Crossref] [PubMed]
- Rampersaud YR, Foley KT, Shen AC, et al. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine (Phila Pa 1976) 2000;25:2637-45. [Crossref] [PubMed]
- Zorn KC, Gofrit ON, Orvieto MA, et al. Da Vinci robot error and failure rates: single institution experience on a single three-arm robot unit of more than 700 consecutive robot-assisted laparoscopic radical prostatectomies. J Endourol 2007;21:1341-4. [Crossref] [PubMed]
- Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 2016;388:1057-66. [Crossref] [PubMed]
- Wren SM, Curet MJ. Single-port robotic cholecystectomy: results from a first human use clinical study of the new da Vinci single-site surgical platform. Arch Surg 2011;146:1122-7. [Crossref] [PubMed]
- Tasci AI, Tufek I, Gumus E, et al. Oncologic results, functional outcomes, and complication rates of robotic-assisted radical prostatectomy: multicenter experience in Turkey including 1,499 patients. World J Urol 2015;33:1095-102. [Crossref] [PubMed]
- Soto E, Lo Y, Friedman K, et al. Total laparoscopic hysterectomy versus da Vinci robotic hysterectomy: is using the robot beneficial? J Gynecol Oncol 2011;22:253-9. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [Crossref] [PubMed]
- Bederman SS, Hahn P, Colin V, et al. Robotic Guidance for S2-Alar-Iliac Screws in Spinal Deformity Correction. Clin Spine Surg 2017;30:E49-53. [Crossref] [PubMed]
- Hyun SJ, Kim KJ, Jahng TA, et al. Minimally Invasive Robotic Versus Open Fluoroscopic-guided Spinal Instrumented Fusions: A Randomized Controlled Trial. Spine (Phila Pa 1976) 2017;42:353-8. [Crossref] [PubMed]
- Kantelhardt SR, Martinez R, Baerwinkel S, et al. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J 2011;20:860-8. [Crossref] [PubMed]
- Keric N, Doenitz C, Haj A, et al. Evaluation of robot-guided minimally invasive implantation of 2067 pedicle screws. Neurosurg Focus 2017;42:E11. [Crossref] [PubMed]
- Kim HJ, Jung WI, Chang BS, et al. A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Robot 2017. [Crossref] [PubMed]
- Kuo KL, Su YF, Wu CH, et al. Assessing the Intraoperative Accuracy of Pedicle Screw Placement by Using a Bone-Mounted Miniature Robot System through Secondary Registration. PLoS One 2016;11:e0153235. [Crossref] [PubMed]
- Lonjon N, Chan-Seng E, Costalat V, et al. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J 2016;25:947-55. [Crossref] [PubMed]
- Macke JJ, Woo R, Varich L. Accuracy of robot-assisted pedicle screw placement for adolescent idiopathic scoliosis in the pediatric population. J Robot Surg 2016;10:145-50. [Crossref] [PubMed]
- Pechlivanis I, Kiriyanthan G, Engelhardt M, et al. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine (Phila Pa 1976) 2009;34:392-8. [Crossref] [PubMed]
- Schizas C, Thein E, Kwiatkowski B, et al. Pedicle screw insertion: robotic assistance versus conventional C-arm fluoroscopy. Acta Orthop Belg 2012;78:240-5. [PubMed]
- Sukovich W, Brink-Danan S, Hardenbrook M. Miniature robotic guidance for pedicle screw placement in posterior spinal fusion: early clinical experience with the SpineAssist. Int J Med Robot 2006;2:114-22. [Crossref] [PubMed]
- Tsai TH, Wu DS, Su YF, et al. A retrospective study to validate an intraoperative robotic classification system for assessing the accuracy of kirschner wire (K-wire) placements with postoperative computed tomography classification system for assessing the accuracy of pedicle screw placements. Medicine (Baltimore) 2016;95:e4834. [Crossref] [PubMed]
- Ringel F, Stuer C, Reinke A, et al. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine (Phila Pa 1976) 2012;37:E496-501. [Crossref] [PubMed]
- Lee KH, Yeo W, Soeharno H, et al. Learning curve of a complex surgical technique: minimally invasive transforaminal lumbar interbody fusion (MIS TLIF). J Spinal Disord Tech 2014;27:E234-40. [Crossref] [PubMed]
- Sharif S, Afsar A. Learning Curve and Minimally Invasive Spine Surgery. World Neurosurg 2018;119:472-8. [Crossref] [PubMed]
- Urakov TM, Chang KH, Burks SS, et al. Initial academic experience and learning curve with robotic spine instrumentation. Neurosurg Focus 2017;42:E4. [Crossref] [PubMed]
- Hu X, Lieberman IH. What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clin Orthop Relat Res 2014;472:1839-44. [Crossref] [PubMed]
- Schatlo B, Martinez R, Alaid A, et al. Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir (Wien) 2015;157:1819-23; discussion 1823.
- Yu E, Khan SN. Does less invasive spine surgery result in increased radiation exposure? A systematic review. Clin Orthop Relat Res 2014;472:1738-48. [Crossref] [PubMed]
- Magerl FP. Stabilization of the lower thoracic and lumbar spine with external skeletal fixation. Clin Orthop Relat Res 1984.125-41. [PubMed]
- Isaacs RE, Podichetty VK, Santiago P, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine 2005;3:98-105. [Crossref] [PubMed]
- Anderson DG, Samartzis D, Shen FH, et al. Percutaneous instrumentation of the thoracic and lumbar spine. Orthop Clin North Am 2007;38:401-8. abstract vii. [Crossref] [PubMed]
- Choi WW, Green BA, Levi AD. Computer-assisted fluoroscopic targeting system for pedicle screw insertion. Neurosurgery 2000;47:872-8. [Crossref] [PubMed]
- Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976) 2007;32:537-43. [Crossref] [PubMed]
- Ringel F, Stoffel M, Stuer C, et al. Minimally invasive transmuscular pedicle screw fixation of the thoracic and lumbar spine. Neurosurgery 2006;59:ONS361-6; discussion ONS366-7.
- Datta G, Gnanalingham KK, Peterson D, et al. Back pain and disability after lumbar laminectomy: is there a relationship to muscle retraction? Neurosurgery 2004;54:1413-20; discussion 1420. [Crossref] [PubMed]
- Gejo R, Matsui H, Kawaguchi Y, et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine (Phila Pa 1976) 1999;24:1023-8. [Crossref] [PubMed]
- Winder MJ, Gilhooly PM. Accuracy of minimally invasive percutaneous thoracolumbar pedicle screws using 2D fluoroscopy: a retrospective review through 3D CT analysis. J Spine Surg 2017;3:193-203. [Crossref] [PubMed]
- Aoude AA, Fortin M, Figueiredo R, et al. Methods to determine pedicle screw placement accuracy in spine surgery: a systematic review. Eur Spine J 2015;24:990-1004. [Crossref] [PubMed]
- Patel RD, Graziano GP, Vanderhave KL, et al. Facet violation with the placement of percutaneous pedicle screws. Spine (Phila Pa 1976) 2011;36:E1749-52. [Crossref] [PubMed]
- Catanzarite T, Tan-Kim J, Whitcomb EL, et al. Ergonomics in Surgery: A Review. Female Pelvic Med Reconstr Surg 2018;24:1-12. [PubMed]
- Kadefors R. Ergonomics: a new frontier in medical engineering. Med Prog Technol 1982;9:149-52. [PubMed]
- Mirbod SM, Yoshida H, Miyamoto K, et al. Subjective complaints in orthopedists and general surgeons. Int Arch Occup Environ Health 1995;67:179-86. [PubMed]
- Waljee JF, Greenfield LJ, Dimick JB, et al. Surgeon age and operative mortality in the United States. Ann Surg 2006;244:353-62. [PubMed]
- Neumayer LA, Gawande AA, Wang J, et al. Proficiency of surgeons in inguinal hernia repair: effect of experience and age. Ann Surg 2005;242:344-8; discussion 348-52. [PubMed]
- O'Neill L, Lanska DJ, Hartz A. Surgeon characteristics associated with mortality and morbidity following carotid endarterectomy. Neurology 2000;55:773-81. [Crossref] [PubMed]
- Hartz AJ, Kuhn EM, Pulido J. Prestige of training programs and experience of bypass surgeons as factors in adjusted patient mortality rates. Med Care 1999;37:93-103. [Crossref] [PubMed]
- Kappert U, Tugtekin SM, Cichon R, et al. Robotic totally endoscopic coronary artery bypass: a word of caution implicated by a five-year follow-up. J Thorac Cardiovasc Surg 2008;135:857-62. [Crossref] [PubMed]
- Hakimi AA, Feder M, Ghavamian R. Minimally invasive approaches to prostate cancer: a review of the current literature. Urol J 2007;4:130-7. [PubMed]
- Advincula AP, Song A. The role of robotic surgery in gynecology. Curr Opin Obstet Gynecol 2007;19:331-6. [Crossref] [PubMed]
- Ponnusamy K, Chewning S, Mohr C. Robotic approaches to the posterior spine. Spine (Phila Pa 1976) 2009;34:2104-9. [Crossref] [PubMed]
- Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author's transl). Z Orthop Ihre Grenzgeb 1982;120:343-7. [Crossref] [PubMed]
- Karikari IO, Isaacs RE. Minimally invasive transforaminal lumbar interbody fusion: a review of techniques and outcomes. Spine (Phila Pa 1976) 2010;35:S294-301. [Crossref] [PubMed]
- Holly LT, Schwender JD, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus 2006;20:E6. [Crossref] [PubMed]
- Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976) 1997;22:667-79; discussion 679-80. [Crossref] [PubMed]
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28:S26-35. [Crossref] [PubMed]
- Foley KT, Lefkowitz MA. Advances in minimally invasive spine surgery. Clin Neurosurg 2002;49:499-517. [PubMed]
- Snyder LA. Integrating robotics into a minimally invasive transforaminal interbody fusion workflow. Neurosurg Focus 2018;45:V4. [Crossref] [PubMed]
- Chenin L, Peltier J, Lefranc M. Minimally invasive transforaminal lumbar interbody fusion with the ROSA(TM) Spine robot and intraoperative flat-panel CT guidance. Acta Neurochir (Wien) 2016;158:1125-8. [Crossref] [PubMed]
- Du JP, Fan Y, Liu JJ, et al. Application of Gelatin Sponge Impregnated with a Mixture of 3 Drugs to Intraoperative Nerve Root Block Combined with Robot-Assisted Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery in the Treatment of Adult Degenerative Scoliosis: A Clinical Observation Including 96 Patients. World Neurosurg 2017;108:791-7. [Crossref] [PubMed]
- Cui GY, Tian W, He D, et al. Effects of robot-assisted minimally invasive transforaminal lumbar interbody fusion and traditional open surgery in the treatment of lumbar spondylolisthesis. Zhonghua Wai Ke Za Zhi 2017;55:543-8. [PubMed]
- Eck JC, Hodges S, Humphreys SC. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg 2007;15:321-9. [Crossref] [PubMed]
- Harmon PH. Anterior excision and vertebral body fusion operation for intervertebral disk syndromes of the lower lumbar spine: three-to five-year results in 244 cases. Clin Orthop Relat Res 1963.107-27. [PubMed]
- Mathews HH, Evans MT, Molligan HJ, et al. Laparoscopic discectomy with anterior lumbar interbody fusion. A preliminary review. Spine (Phila Pa 1976) 1995;20:1797-802. [Crossref] [PubMed]
- Regan JJ, Yuan H, McAfee PC. Laparoscopic fusion of the lumbar spine: minimally invasive spine surgery. A prospective multicenter study evaluating open and laparoscopic lumbar fusion. Spine (Phila Pa 1976) 1999;24:402-11. [Crossref] [PubMed]
- Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22:691-9; discussion 700. [Crossref] [PubMed]
- Saraph V, Lerch C, Walochnik N, et al. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J 2004;13:425-31. [Crossref] [PubMed]
- Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J 2002;2:216-23. [Crossref] [PubMed]
- Lee JY, Bhowmick DA, Eun DD, et al. Minimally invasive, robot-assisted, anterior lumbar interbody fusion: a technical note. J Neurol Surg A Cent Eur Neurosurg 2013;74:258-61. [Crossref] [PubMed]
- Troude L, Boissonneau S, Malikov S, et al. Robot-assisted multi-level anterior lumbar interbody fusion: an anatomical study. Acta Neurochir (Wien) 2018;160:1891-8. [Crossref] [PubMed]
- Beutler WJ, Peppelman WC Jr, DiMarco LA. The da Vinci robotic surgical assisted anterior lumbar interbody fusion: technical development and case report. Spine (Phila Pa 1976) 2013;38:356-63. [Crossref] [PubMed]


