
Burkholderia cepacia as a cause of pyogenic spondylodiscitis in immunocompetent patients: a single-institution case series and literature review
Introduction
Burkholderia cepacia, a rare human pathogen that was most likely involved in opportunistic infections in immunocompromised hosts, mainly in patients with cystic fibrosis and chronic granulomatous disease (1,2).
Burkholderia cepacia is established to be the cause of pyogenic spondylodiscitis in only seven cases in the medical literature (3-8). Cervical spondylodiscitis was described after rhinoplasty (6) and in an IV drug user (5), thoracic osteomyelitis in a healthy farmer (3), and lumbar osteomyelitis after slippage on an icy road (7) and after laparoscopic cholecystectomy (4). Four of these cases demonstrated complete resolution with adequate antibiotic therapy while the case of thoracic osteomyelitis succumbed due to septic shock and respiratory failure (3). Two more cases of hematogenous pyogenic vertebral osteomyelitis due to B. cepacia were described in a 12-year retrospective study done in Taiwan (8).
Here, we report the cases of four patients who were diagnosed with Burkholderia cepacia spondylodiscitis during two years period and were successfully managed by appropriate antimicrobial treatment after open surgical biopsy for microbiological identification and debridement. The pathogen was identified using VITEK® 2 System (Biomerieux).
Case presentation
Case 1
A 60-year-old Iraqi female previously healthy who started to complain of sudden onset localized low back pain that started 1 month before admission after she underwent laparoscopic cholecystectomy at another hospital in Iraq, pain is aggravated with motion of the back, not relieved by over the counter painkillers and NSAIDs, and she denied associated fever or chills. Because of the patient's nonresponse to treatment, she decided to seek medical advice at our facility. Physical exam was unremarkable except for local tenderness at L5–S1 Level. Her labs showed mainly normal WBC of 6,600 cu.mm (normal: 4,000–11,000 cu.mm) and elevated CRP of 37 mg/L (normal <5 mg/L) and ESR of 80 mm/hr (normal <20 mm/h) compatible with infection. Lumbar MRI showed findings suggestive of spondylodiscitis at the L5–S1 level.
Fluoroscopy guided L5–S1 disc biopsy was performed but the culture was negative. The decision was made to proceed with open surgical bone and disc biopsy with debridement through a posterior hemilaminectomy approach. Specimens sent for microbiological identification. A bone biopsy confirmed the growth of B. cepacia sensitive to Ceftazidime, Amikacin, Trimethoprim/Sulfamethoxazole, Imipenem, Piperacillin/Tazobactam.
The Patient was treated empirically with Vancomycin and Ceftazidime and was switched to Ceftazidime 2 g IV TID after bacterial identification for a course of 4 weeks. At the end of the course, she became pain-free and her CBCD and CRP level were normal. She remained asymptomatic on long-term follow up of 6 months.
Case 2
A 64-year-old Iraqi female who presented for sudden onset localized low back pain starting 2 months before admission, aggravated with motion of the back and interferes with walking, she is known to be hypertensive and underwent posterior approach laminectomy with transpedicular screws fixation L2–L3–L4 2 years ago at another hospital in Iraq. Her neurologic exam was normal. Localized tenderness was noted at L4-L5 level. Her labs showed a normal WBC of 9,900 cu.mm (normal: 4,000–11,000 cu.mm), elevated CRP of 31 mg/L (normal <5 mg/L) and ESR of 55 mm/hr (normal <20 mm/h). MRI Lumbar spine showed findings suggestive of spondylodiscitis at the L4–L5 level. Posterior approach L4-L5 hemilaminectomy was done to have bone and disc culture followed by removal of the previously inserted screws and rods. Disc culture confirmed the growth of B. cepacia sensitive to Ceftazidime, Cefepime, and Meropenem.
Immediately after the surgery, the patient was treated empirically with Vancomycin and Ceftazidime and was switched to Meropenem 2 g IV TID for 6 weeks. At the end of the course, she became pain-free and her CBCD and CRP level were normal. She remained asymptomatic on long-term follow up of 6 months.
Case 3
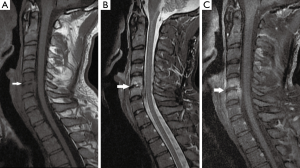
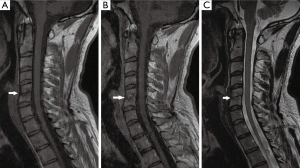
A 34-year-old Lebanese athletic (Kickboxer) male, previously healthy, his history is remarkable for submucous resection of the nose 1 month at another hospital in Lebanon before admission. His major complaint was a gradual onset of severe neck pain radiating to left shoulder over 1 week that was not alleviated by NSAIDS and over the counter medications. On examination, he was non-feverish, systemically well with no neurological signs. Neck movements were limited by pain and no focal spinal tenderness was found. His labs showed a normal WBC of 8,200 cu.mm (normal: 4,000–11,000 cu.mm), mildly increased CRP of 6.76 mg/L (normal <5 mg/L) and a normal ESR level of 14 mm/hr (normal <20 mm/h). Cervical spine MRI showed findings compatible with C5–C6 spondylodiscitis (Figure 1). The patient underwent an anterior approach C5–C6 disc biopsy. Disc cultures confirmed the growth of B. cepacia sensitive to Meropenem. He was started empirically on Ceftazidime and vancomycin and was switched to Meropenem 2 g IV TID for 4 weeks. At the end of the course, he became pain-free and his CBCD and CRP level were normal. He remained asymptomatic on long-term follow up of 6 months. A postoperative cervical spine MRI done 3 months after surgery is demonstrated in Figure 2.
Case 4
A 51-year-old Iraqi male, previously healthy who underwent posterior approach lumbar laminectomy L4 4 months ago at another hospital in Iraq. After the surgery, he noticed the persistence of his back pain. And sought medical advice at our hospital. His labs showed a normal WBC of 12,000 cu.mm (normal: 4,000–11,000 cu.mm), an increased CRP of 31 mg/L (normal <5 mg/L) and an ESR level of 42 mm/hr (normal <20 mm/h). MRI lumbar spine showed findings suggestive of L4-L5 Spondylodiscitis. Fluoroscopy guided L4-L5 disc biopsy was performed but the culture was negative. Open surgical disc biopsy through a posterior hemilaminectomy approach and debridement were done. He was started empirically on Ceftazidime and Vancomycin. Disc culture confirmed the presence of B. cepacia sensitive only to Meropenem. The Treatment was switched to Meropenem 2 g IV TID for 4 weeks. At the end of the course, he became pain-free and his CBCD and CRP level were normal. He remained asymptomatic on long-term follow up of 6 months.
Concerning case 3 and 4, antimicrobial susceptibility testing was done and interpreted as guided by CLSI (Clinical and Laboratory Standards Institute) only for the following antibiotics: Ceftazidime, Meropenem, Minocycline, Levofloxacin, Bactrim (TMP/SMX) (9).
Discussion
Spondylodiscitis, an infection of the intervertebral disc and adjacent vertebrae which usually occurs through hematogenous spread, represents around 3–5% of all cases of osteomyelitis and predominantly affects people over 50 years of age with a male preponderance (male to female ratio of 1.5–2:1) preferentially at the lumbar level (10). The most frequent causative pathogen of pyogenic spondylodiscitis is Staphylococcus aureus, mostly methicillin-sensitive strains, in about 50% of cases (20–84%), followed by gram-negative rods (7–33%), Coagulase-negative Staphylococci (5–16%), Streptococci and Enterococci (5–20%), and Anaerobes (3%) (11). Often, spondylodiscitis diagnosis is delayed to advanced stages due to the lack of specificity of signs and symptoms rendering the diagnosis challenging and increasing the risk of comorbidities and complications (12).
Only seven cases were described in the literature about B. Cepacia Spondylodiscitis in immunocompetent patients: cervical osteomyelitis after rhinoplasty (6), cervical osteomyelitis in an intravenous-drug abuser (4), thoracic osteomyelitis in a healthy adult with negative past surgical history (3), and lumbar osteomyelitis in an elderly patient (7) and lumbar osteomyelitis after cholecystectomy (4). Two more cases of hematogenous pyogenic vertebral osteomyelitis due to B. cepacia were described in a 12-year retrospective study done in Taiwan (8).
Burkholderia cepacia, previously called Pseudomonas cepacia when it was first isolated in the 1950s as the cause of onion rot, it is a gram-negative motile aerobic bacillus that has a ubiquitous distribution in water, soil, and plants (3,5,7). It is an important pathogen that infects patients particularly with cystic fibrosis (CF), chronic granulomatous diseases, and immunocompromised patients (1,2). It has been rarely suspected as a cause of human infections, most commonly nosocomial infections (13).
Water valves, distilled water, water baths, nebulizers, dialysis fluids and machines, contaminant disinfectants, solutions and intravenous fluids, catheters, blood gas analyzers, thermometers, and ventilator temperature sensors were the most common sources where the pathogen was isolated from in the hospital setting (7). B. cepacia is recognized as difficult-to-treat pathogen due to its broad antimicrobial resistance pattern caused by several innate (innate decreased permeability of the bacterial membrane, efflux pumps, beta-lactamases) and acquired mechanisms (emergence of hypermutable bacteria, induction of cross-resistance among different classes of antibiotics) (14).
Co-trimoxazole (trimethoprim/sulfamethoxazole) was considered the drug of choice. However, allergic or hypersensitivity reactions, intolerance, and resistance can occur with patients treated with Co-trimoxazole (14). Most active alternative options are Ceftazidime, Piperacillin, Meropenem, and Minocycline (14).
Percutaneous biopsy techniques (CT-guided and fluoroscopy-guided needle biopsy) are safe and minimally invasive diagnostic procedures to identify pathogens in lumbar spondylodiscitis with variable rates of success. However, an open biopsy should be considered if the percutaneous biopsy is negative or non-diagnostic (15).
Surgical treatment is considered with failure of conservative treatment and is mandatory in cases of advanced stage spondylodiscitis, neurologic deficits, progressive septicemia, and/or progressive instability or deformity (16).
Anterior open debridement and instrumentation were classically the preferred treatment, due to its good outcome and low intraoperative complication rates. A single anterior approach is particularly feasible in cervical spondylodiscitis. A combined anterior and posterior approach is appropriate in cases of extensive involvement requiring multiple corpectomies. A posterior transforaminal or posterior interbody debridement and fusion can be considered in cases in which an anterior approach is contraindicated mainly in lumbar discitis and/or minimal vertebral involvement (16). Minimally invasive debridement and instrumentation may lead to immediate pain reduction and good clinical results in patients with comorbid medical problems and could be considered as a safe alternative to conventional open surgery (17). Overall benefits of minimally invasive debridement and instrumentation include less blood loss, less spread of infection, less wound infection, and may ultimately lead to more rapid fusion and therefore to faster recovery. As for percutaneous instrumentation, it may only be a valuable option in patients showing no space-occupying abscess and lacking deformity and/or pathologic fracture (16).
To note that three of our reported cases developed B. cepacia spondylodiscitis postoperatively (1–4 months period). Which implies that the pathogen is more likely acquired in the hospital setting. Therefore, better decontamination, sterilization and disinfection methods should be applied to provide an optimal environmental hygiene to prevent the acquisition of such pathogen. This also highlights the need for optimization of patient risk factors preoperatively to prevent surgical site infections mainly by the following measures: encouraging smoking cessation, strict blood glucose control, weight loss, steroid cessation or minimization if possible, nutritional optimization, MRSA screening 30 days prior to planned procedures with a 5-day decolonization treatment using mupirocin if necessary, and Chlorhexidine gluconate (CHG) bathing for 5 days prior to surgery, antibiotic administration 1 hour prior to incision, and skin preparation with CHG (18). In addition, we should mention the role of intrawound vancomycin application, which was proven safe and effective in reducing SSI incidence to approximately one-third (OR 0.39) of that when vancomycin was not applied, in the most recent meta-analysis (19).
Also, two of our cases demonstrated explicit sensitivity of the pathogen only to Meropenem which confirms the broad antimicrobial resistance of this type of infection.
The major characteristics of every patient as well as from similar cases in the literature are shown in Table 1.
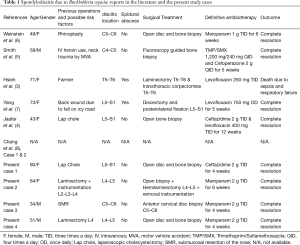
Full table
Our reported cases developed good clinical, and biological improvement after the initiation of appropriate antimicrobial treatment based on cultures and antimicrobial susceptibility testing results.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Bottone EJ, Douglas SD, Rausen AR, et al. Association of Pseudomonas cepacia with chronic granulomatous disease. J Clin Microbiol 1975;1:425-8. [PubMed]
- Tablan OC, Chorba TL, Schidlow DV, et al. Pseudomonas cepacia colonization in patients with cystic fibrosis: Risk factors and clinical outcome. J Pediatr 1985;107:382-7. [Crossref] [PubMed]
- Hsieh CT, Hsu SK, Chang CJ. Thoracic Vertebral Osteomyelitis Caused by Burkholderia cepacia in an Immunocompetent Adult. Surg Infect (Larchmt) 2013;14:476-9. [Crossref] [PubMed]
- Jaafar D, Rizkallah M, Atallah F, et al. Lumbar Spondylodiscitis Caused by Burkholderia cepacia in a Previously Healthy Patient. Case Rep Orthop 2017;2017:1396950.
- Smith MA, Trowers NR, Klein RS. Cervical osteomyelitis caused by Pseudomonas cepacia in an intravenous-drug abuser. J Clin Microbiol 1985;21:445-6. [PubMed]
- Weinstein L, Knowlton CA, Smith MA. Cervical osteomyelitis caused by Burkholderia cepacia after rhinoplasty. J Infect Dev Ctries 2008;2:76-7. [PubMed]
- Yang BH, Lee MS, Lee JH, et al. Pyogenic spondylitis in a healthy adult caused by Burkholderia cepacia. Infect Chemother 2008;40:233-6. [Crossref]
- Chang WS, Ho MW, Lin PC, et al. Clinical characteristics, treatments, and outcomes of hematogenous pyogenic vertebral osteomyelitis, 12-year experience from a tertiary hospital in central Taiwan. J Microbiol Immunol Infect 2018;51:235-42. [Crossref] [PubMed]
- CLSI (2017) M100: Performance Standards for Antimicrobial Susceptibility Testing, 27th Edition. Available online: accessed January 2017.http://file.qums.ac.ir/repository/mmrc/clsi%202017.pdf
- Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010;65:ii11-24. [Crossref] [PubMed]
- Fantoni M, Trecarichi EM, Rossi B, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci 2012;16 Suppl 2:2-7. [PubMed]
- Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: An overview. J Infect Public Health 2010;3:5-16. [Crossref] [PubMed]
- Pallent LJ, Hugo WB, Grant DJ, et al. Pseudomonas cepacia as contaminant and infective agent. J Hosp Infect 1983;4:9-13. [Crossref] [PubMed]
- Avgeri SG, Matthaiou DK, Dimopoulos G, et al. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int J Antimicrob Agents 2009;33:394-404. [Crossref] [PubMed]
- Nam KH, Song GS, Han IH, et al. Diagnostic Value of Biopsy Techniques in Lumbar Spondylodiscitis: Percutaneous Needle Biopsy and Open Biopsy. Korean J Spine 2011;8:267-71. [Crossref] [PubMed]
- Lener S, Hartmann S, Barbagallo GMV, et al. Management of spinal infection: a review of the literature. Acta Neurochir (Wien) 2018;160:487-96. [Crossref] [PubMed]
- Deininger MH, Unfried MI, Vougioukas VI, et al. Minimally invasive dorsal percutaneous spondylodesis for the treatment of adult pyogenic spondylodiscitis. Acta Neurochir (Wien) 2009;151:1451-7. [Crossref] [PubMed]
- Nasser R, Kosty JA, Shah S, et al. Risk Factors and Prevention of Surgical Site Infections Following Spinal Procedures. Global Spine J 2018;8:44S-8S. [Crossref] [PubMed]
- Tailaiti A, Shang J, Shan S, et al. Effect of intrawound vancomycin application in spinal surgery on the incidence of surgical site infection: a meta-analysis. Ther Clin Risk Manag 2018;14:2149-59. [Crossref] [PubMed]


