Accuracy of cortical bone trajectory screw placement in midline lumbar fusion (MIDLF) with intraoperative cone beam navigation
Introduction
Minimally invasive surgery (MIS) techniques have been increasingly utilized to perform lumbar fusions. Traditionally, pedicle screws provide the robust initial stability required for arthrodesis constructs (1). Pedicle screw fixation has been the gold-standard technique for lumbar spine stabilization due to its superior biomechanical strength compared to alternative forms of fixation. Although pedicle screw constructs are often associated with excellent surgical outcomes, there has been concern about the incidence of superior articulating facet violation due to screw proximity to the joint and the amount of soft tissue dissection required for screw placement (2).
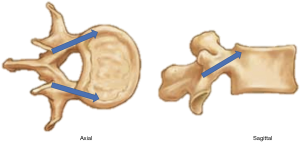
Over recent years, the cortical bone trajectory (CBT) was developed as a minimally invasive screw option that aims to improve fixation by targeting higher density cortical bone. Unlike traditional pedicle screws that begin at the transverse process-superior articular facet junction and progress lateral-to-medial in the axial plane, CBT screws begin at the superolateral aspect of the pars (Figure 1) (3). In the axial plane, the medial to lateral trajectory allows for preservation of lateral paraspinal muscles and their innervation, as well as possibly decreasing the risk of neurologic injury (4).
Traditionally, CBT screws are inserted under fluoroscopic guidance with lengths of approximately 25–30 mm. However, in an effort to reduce surgeon fatigue, reduce surgeon and staff radiation exposure, increase screw length, and improve accuracy of screw placement, CBT screws may be instrumented with intraoperative navigation. To date, there is no study assessing the safety and efficacy of CBT screw placement under the guidance of intraoperative cone beam computed tomography (CT). The purpose of the current study was to evaluate the safety and accuracy of CBT screw placement using intraoperative CT navigation.
Methods
Study design
This retrospective study was conducted at a single, academic medical center. All procedures were performed by one of three fellowship-trained orthopaedic spine surgeons (JL Gum, M Djurasovic, CH Crawford). All aspects of this study were reviewed and approved by the university’s institutional review board. A retrospective chart review was performed on all patients who underwent spine surgery with use of intraoperative cone beam CT navigation (StealthStation®, Medtronic Sofamor Danek.; Minneapolis, Minnesota, USA) from the time period of May 1st, 2016 to May 1st, 2018. All patients without post-instrumentation axial imaging were excluded from the study. Over that period, 134 patients underwent CBT fixation under intraoperative navigation. In total, 618 screws were included in the analysis. Standard demographic information (age, gender, diagnosis), body mass index (BMI), smoking status, presence of diabetes or steroid medication use and surgical data was collected from the electronic medical record system. CT scans were obtained at the same institution using the same technique in each patient. One-millimeter-thick, continuous, non-overlapping axial slices were obtained, in addition to sagittal and coronal multi-planar reconstructions. Additionally, intraoperative data, including operative reports and technical notes, were reviewed. Three independent fellowship-trained spine surgeons (JL Laratta, JN Shillingford, AJ Pugely) analyzed intraoperative fluoroscopy findings as well as CT scans to assess placement of CBT screws. To eliminate potential bias, the independent surgeons who reviewed the screws for accuracy were not the surgeon-of-record on the respective cases. CT analysis consisted of 2D and 3D reconstructions created with VitreaCore software (Vital Images; Minnetonka, Minnesota, USA).
Navigation-assisted surgical technique
The patient was positioned prone on a 6-post Jackson frame with all bony prominences padded and abdomen free from pressure. A surgical time out was performed in accordance to World Health Organization policies. The patient was given antibiotic prophylaxis based on weight. The incision was localized with a spinal needle. A 5-cm incision (for a single level procedure) was made in the midline over the interspace. Incision was carried through the subcutaneous tissue to the fascia, which was split longitudinally. Subperiosteal dissection continued along the spinous process and lamina. The subperiosteal dissection did not extend lateral to the inferior articular process of the cephalad vertebra. Inferior facetectomy at the caudad level was performed intermittently based on the operative surgeon’s preference.
A reference frame was clamped on the cephalad spinous process with careful attention that the orientation of the frame would not interfere with planned CBT screw start points and trajectories. The O-arm was used to obtain an intraoperative CT scan. The O-arm obtained images in low-dose protocol in an effort to minimize radiation the patient. The images were transferred to the StealthStation. Similar to fluoroscopic technique, the CBT screw starting point began 1mm medial to the lateral aspect of the pars interarticularis and 1mm inferior to the transverse process (which projected at the 5 o’clock orientation in the left pedicle and 7 o’clock orientation in the right pedicle). A navigated drill was used to create the first 5 mm of the cortical track. A navigated tap was then inserted into the track and used to create the medial-to-lateral and inferior-to-superior CBT. The tap was advanced until reaching the lateral cortex of the vertebral body or superior endplate. Care was taken to ensure that endplate integrity was not violated. Given the cortical trajectory, screws were tapped line-to-line to prevent iatrogenic fracture and loss of fixation. Screws were placed under navigated guidance and sizes were based on reverse projections on the StealthStation. Most screws ranged from 30–40 mm in length and 4.5–5.5 mm in diameter. The screw diameter was determined by the surgeon based on the particular patient’s corridor for the cortical trajectory. All screws were uniformly pitched. Reduction screws were not utilized in the current study. Where applicable, reduction was performed with postural patient positioning, efficient disc preparation, and rod reducers. In revision cases for adjacent level pathology, CBT screws were placed in the adjacent level and connected to the existing construct with domino connectors.
Screw analysis
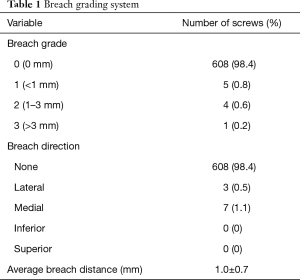
Assessment of screw placement accuracy was based on post-instrumentation CT scans. All scans were uploaded from local imaging systems into the VitreaCore software. Screw analysis was performed as originally described by Laratta and colleagues (5). The VitreaCore software allowed for both 2D and 3D analyses of screw placement. Additionally, by altering the CT gantry on both sagittal and axial planes, the path of the screw could be tracked down its central axis. This technique allowed for a 360-degree visualization around the axis of the screw and a rigorous assessment of cortical breach. A screw breach was defined as any part of the screw threads crossing outside the cortex in any direction (superior, inferior, medial, lateral). When breach was determined to be present, the distance was measured at its greatest point. Breaches were graded based on severity (Table 1).
Full table
Statistical analysis
Unadjusted univariate analysis was performed to determine the mean differences between measurements utilizing independent student t-test for continuous data and Chi-squared or Fisher’s exact testing for categorical variables. Findings were considered statistically significant when the P value was <0.05. Analysis was conducted using IBM SPSS Statistics version 24.
Results
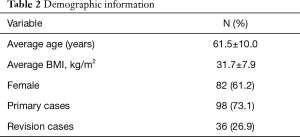
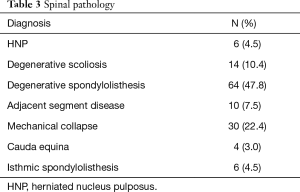
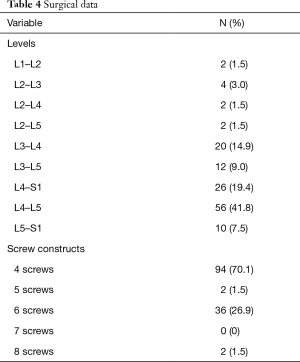
Six hundred and eighteen CBT screws were analyzed in 134 patients, consisting of 82 females (61.2%) and 52 males (38.8%). Patient characteristics are reported in Table 2. The mean age at the time of surgery was 61.5±10.0 years, and mean BMI was 31.7±7.9 kg/m2. The mean operative duration was 135.3±17.3 minutes. Nearly three-quarters of patients had no history of lumbar surgery (73.1%). The most common diagnoses (Table 3) included degenerative spondylolisthesis (47.8%), mechanical collapse with foraminal stenosis (22.4%), degenerative scoliosis (10.4%), and adjacent segment disease (7.5%). Surgical data are reported in Table 4. Most screw constructs included one level and four CBT screws (70.1%). The most commonly instrumented level was L4–L5 (41.8%).
Full table
Full table
Full table
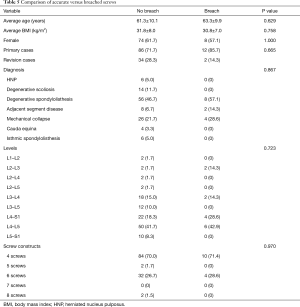
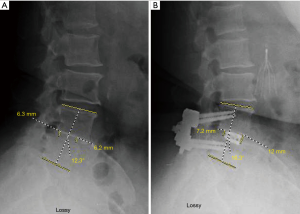
Intraoperative CT scans were available for evaluation of every CBT screw placed under navigated guidance. The mean screw length and diameter was 40.0±2.6 and 5.2±0.4 mm, respectively. The blinded clinical evaluations of screw placement indicated that ten violations of the cortex occurred with an average breach distance of 1.0±0.7 mm (Table 1). Three breaches were lateral (0.5%) and seven were medial (1.1%). There were five breaches (0.8%) over 1 mm and only 1 breach over 3 mm. The overall navigated screw accuracy rate was 98.3%. The accuracy to within 1 mm of error was 99.2%. There were no significant differences (P>0.5) in accurate versus breached screws (Table 5). There were no intra-operative mechanical, neurologic, vascular, or visceral complications related to the placement of the CBT screws. A case of degenerative spondylolisthesis treated with a midline lumbar interbody fusion with CBT screws is shown in Figure 2.
Full table
Discussion
Fusion of the lumbosacral spine is commonly indicated after failure of conservative management for a number of degenerative and traumatic spinal conditions (6). CBT screws have been recently described as a method of lumbosacral fixation with increased cortical bone purchase. Unlike the traditional lateral-to-medial trajectory of pedicle screws that traverse intrapedicular, cancellous bone, CBT screws have a medial-to-lateral trajectory with an entirely cortical course (3,4). Moreover, because CBT screws are placed through a midline incision without dissection lateral to the facet, there is decreased paraspinal muscle injury and denervation, which could potentially allow for less pain, quicker recovery, and less adjacent level disease (7,8).
There are a number of biomechanical studies that compare the stability of CBT screws with traditional pedicle screws. In a cadaveric study, Perez-Orribo and colleagues reported that bilateral CBT screw-rod constructs provided about the same stability as pedicle screw-rod constructs (9). A follow-up cadaveric study reported that CBT screws have superior resistance to craniocaudal toggling compared with traditional pedicle screws (10). Unfortunately, the clinical evidence supporting CBT screws is still rather limited (11,12).
Traditionally, CBT screws are meticulously placed under fluoroscopic guidance due to the complex 3D anatomy of the lumbar spine and surrounding neurologic structures. Unfortunately, the technique of screw placement with repeated static and live fluoroscopic images exposes both patient and surgeon to radiation. Additionally, fluoroscopic CBT screw placement can be time consuming and surgeon dependent. Empirically in our practice, intraoperative CT navigation-guided CBT screw placement has reduced radiation exposure to the surgeon, decreased operative time, and improved accuracy.
To date, this is the first study to examine the safety and accuracy of CBT screw placement with intraoperative CT navigation. In a previous study, Le et al. compared robotic and fluoroscopic guidance for CBT screw placement. The authors reported a 13.1% and 4.7% rate of clinically unacceptable CBT screw placement with fluoroscopy and robotic guidance, respectively (13). In our series, 618 CBT screws in 134 patients were evaluated in our series. The overall accuracy rate for intraoperative CT-guided CBT screw placement was 98.3%, and over 99.2% accuracy within 1 mm. There were no significant differences between accurate and breached screws (P>0.5). The breaches represent a deviation from the navigated guidance plan. It is possible that during the drilling or instrumentation process, the reference frame was inadvertently violated causing loss of correct trajectory. Most breaches were in the lateral direction—which is likely a result of the surgeons inadvertently favoring a more lateral trajectory in order to avoid a neurologically significant medial breach. Regardless, there were no intra-operative mechanical, neurologic, vascular, or visceral complications related to the placement of the CBT screws. Similarly, other studies showed no difference in complications with CBT screws compared to more traditional methods of lumbar fixation (14,15).
Although the current study is retrospective in nature, it is the first study of its kind to assess the safety and efficacy of CBT screw placement under intraoperative navigated-guidance. Before adopting new surgical techniques, it is important to critically assess the application of the technology prior to widespread usage, and the current study provides this data. Despite being the only series assessing the accuracy of intraoperatively-navigated CBT screws, our study may benefit from a larger sample size gathered over several institutions for more rigorous assessment and improved external validity.
In conclusion, intraoperative cone beam CT navigation for CBT screw placement is a safe and effective way to achieve lumbar fixation with over 98–99% accuracy. There were no complications directly related to the navigated placement of the CBT screws in this series.
Acknowledgments
None.
Footnote
Conflicts of Interest: JL Laratta—consulting (Spineart, Evolution Spine, Gerson Lehrman Group), royalties (Spineart, Evolution Spine), Intellectual Property (Combination Biologic, ICBG/BMAC system), grants (Medtronic, Nuvasive, Orthopaedic Science Research Foundation, Orthopaedic Research and Education Foundation), editorial board (Spine, Global Spine Journal, Journal of Spine Surgery). JL Gum—consulting (Medtronic, Depuy, Alphatec, Stryker, Acuity, K2M, NuVasive, Pacmed), royalties (Acuity), editorial board (American Journal of Orthopedics), grants (Fischer Owen Fund), research support (Integra, Intellirod Spine Inc., Pfizer, International Spine Study Group Foundation, NuVasive). M Djurasovic—consulting (Medtronic), royalties (Medtronic, NuVasive), research support (NuVasive, Pfizer, Norton Healthcare), speaker (Medtronic). CH Crawford—consulting (Alphatec, Medtronic), speaker (Norton Hospital), research funding (Norton Hospital). The other authors howe no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval for this study was granted by the University of Louisville IRB (#14.0803). Study outcomes will not affect the future management of patients. Informed consent forms were not required from patients due to data being collected from a hospital electronic medical record system. All patient’s personal data have been secured.
References
- Boucher HH. A method of spinal fusion. J Bone Joint Surg Br 1959;41-B:248-59. [Crossref] [PubMed]
- Lowery GL, Kulkarni SS. Posterior percutaneous spine instrumentation. Eur Spine J 2000;9 Suppl 1:S126-30. [Crossref] [PubMed]
- Santoni BG, Hynes RA, McGilvray KC, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J 2009;9:366-73. [Crossref] [PubMed]
- Matsukawa K, Yato Y, Nemoto O, et al. Morphometric measurement of cortical bone trajectory for lumbar pedicle screw insertion using computed tomography. J Spinal Disord Tech 2013;26:E248-53. [Crossref] [PubMed]
- Laratta JL, Shillingford JN, Lombardi JM, et al. Accuracy of S2 Alar-Iliac Screw Placement Under Robotic Guidance. Spine Deform 2018;6:130-6. [Crossref] [PubMed]
- Kaiser MG, Eck JC, Groff MW, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology. J Neurosurg Spine 2014;21:2-6. [Crossref] [PubMed]
- Chin KR, Pencle FJR, Coombs AV, et al. Clinical Outcomes With Midline Cortical Bone Trajectory Pedicle Screws Versus Traditional Pedicle Screws in Moving Lumbar Fusions From Hospitals to Outpatient Surgery Centers. Clin Spine Surg 2017;30:E791-7. [Crossref] [PubMed]
- Lee GW, Son JH, Ahn MW, et al. The comparison of pedicle screw and cortical screw in posterior lumbar interbody fusion: a prospective randomized noninferiority trial. Spine J 2015;15:1519-26. [Crossref] [PubMed]
- Perez-Orribo L, Kalb S, Reyes PM, et al. Biomechanics of lumbar cortical screw-rod fixation versus pedicle screw-rod fixation with and without interbody support. Spine (Phila Pa 1976) 2013;38:635-41. [Crossref] [PubMed]
- Baluch DA, Patel AA, Lullo B, et al. Effect of physiological loads on cortical and traditional pedicle screw fixation. Spine (Phila Pa 1976) 2014;39:E1297-302. [Crossref] [PubMed]
- Gonchar I, Kotani Y, Matsumoto Y. Cortical bone trajectory versus percutaneous pedicle screw in minimally invasive posterior lumbar fusion. Spine J 2014;14:S114-5. [Crossref]
- Rodriguez A, Neal MT, Liu A, et al. Novel placement of cortical bone trajectory screws in previously instrumented pedicles for adjacent-segment lumbar disease using CT image-guided navigation. Neurosurg Focus 2014;36:E9. [Crossref] [PubMed]
- Le X, Tian W, Shi Z, et al. Robot-Assisted Versus Fluoroscopy-Assisted Cortical Bone Trajectory Screw Instrumentation in Lumbar Spinal Surgery: A Matched-Cohort Comparison. World Neurosurg 2018;120:e745-51. [Crossref] [PubMed]
- Sakaura H, Miwa T, Yamashita T, et al. Posterior lumbar interbody fusion with cortical bone trajectory screw fixation versus posterior lumbar interbody fusion using traditional pedicle screw fixation for degenerative lumbar spondylolisthesis: a comparative study. J Neurosurg Spine 2016;25:591-5. [Crossref] [PubMed]
- Takenaka S, Mukai Y, Tateishi K, et al. Clinical Outcomes After Posterior Lumbar Interbody Fusion: Comparison of Cortical Bone Trajectory and Conventional Pedicle Screw Insertion. Clin Spine Surg 2017;30:E1411-8. [Crossref] [PubMed]