
Anterior lumbar interbody fusion in a lateral decubitus position: technique and outcomes in obese patients
Introduction
Obesity, which affects over one-quarter of adults in Australia (1) and over one-third of adults in the United States, is associated with higher rates of symptomatic disc degeneration (2). Performing multilevel lumbar interbody fusion (LIF) surgery in obese patients is problematic. Posterior approaches [posterior LIF (PLIF) or transforaminal LIF (TLIF)] in the prone position have major positioning and anaesthetic risks, lateral approaches [lateral LIF (LLIF)] do not allow access to L5/S1 disc level and anterior retroperitoneal or transperitoneal supine approaches [anterior LIF (ALIF)] are associated with the risks of visceral and vascular injuries (3-5). Combined approaches to the lumbar and lumbosacral disc levels require repositioning the patient.
Modified ALIF via an anterolateral retroperitoneal approach in the lateral decubitus position permits access to L3/4, L4/5, and L5/S1 levels without repositioning the patient. Indications for this lateral-position ALIF are similar to those for PLIF/TLIF, LLIF, and ALIF for treating severe discogenic pain, low-grade spondylolisthesis, and deformity. However, lateral ALIF avoids the limitations of LLIF and ALIF and potentially allows high body mass index (BMI) patients to be treated safely. The lateral position utilizes gravity to help retract the abdominal contents away from the surgical site and enables an oblique approach with minimal additional retraction. A vascular surgeon and a spine surgeon then use standard techniques for lateral ALIF.
The aims of this study were to report our initial experience with lateral ALIF in obese patients and describe modifications of existing lateral and anterior techniques for lateral ALIF.
Methods
Study design
In this study, we retrospectively analysed data collected prospectively for the first 30 consecutive patients treated with lateral ALIF by a single spine surgeon and two vascular surgeons from June 2014 to May 2016. All patients were followed for a minimum of 24 months after surgery. Institutional ethics approval was obtained from the Epworth Hospital Research and Development Department.
Inclusion and exclusion criteria
The inclusion criteria for this study were as follows: (I) over 18 years of age; (II) primary single-, two- or three-level symptomatic degenerative disc disease (DDD) or grade 1 degenerative spondylolisthesis at L5/S1, with or without pathology at L3/4 and/or L4/5; (III) failure of more than 6 months of nonsurgical treatment with physical therapy, analgesics, weight-loss programs, and epidural steroid injections; and (IV) relative contraindication to a supine-position midline anterior retroperitoneal approach because of obesity (BMI >30 kg/m2) or extensive previous abdominal surgery.
The exclusion criteria were as follows: (I) symptomatic DDD at L3/4 or L4/5 without pathology at L5/S1; (II) bony lateral recess stenosis; (III) herniated nucleus pulposus with sequestrated free fragments; (IV) extensive previous retroperitoneal surgery, including laparoscopic inguinal hernia mesh repair; (V) retroperitoneal radiation exposure; (VI) left abdominal wall stoma; and (VII) significant vascular pathology, such as a dual inferior vena cava (IVC) or an aortic or iliac artery aneurysm.
Preoperative investigations
These investigations were performed before surgery: (I) flexion and extension radiographs to assess stability at the index disc level(s); (II) magnetic resonance imaging (MRI) to identify neural compression; (III) computed tomography (CT) to evaluate facet arthropathy; (IV) isotope-based bone scan to identify facet and disc pathology; (V) provocative discography to assess discogenic pain; and (VI) bone density (DEXA) scan to detect osteopenia or osteoporosis.
Vascular anatomy at each target disc level was assessed using the MRI and/or CT images. For the L3/4 and L4/5 disc levels, the following were evaluated: (I) position of the aorta and left common iliac artery and size of the aorto-psoas window; (II) presence of left-sided accessory renal arteries; (III) position and size of the segmental lumbar vessels; (IV) position and size of the ascending lumbar and iliolumbar vein(s); and (V) presence of any iliocaval venous anomalies (such as a left-sided or dual IVC). For the L5/S1 disc level, the following were determined: (I) location of the median sacral vessels and (II) location of the left common iliac vein and artery. With a lumbosacral transitional vertebra, the vascular anatomy may be similar to that seen at L4/5 rather than the usual L5/S1 anatomy; if so, the surgical approach was modified accordingly.
Surgical technique
Patient positioning and disc level marking
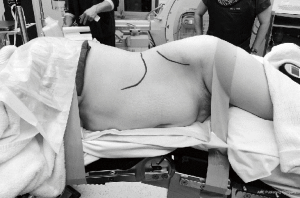
The patient was placed in the right lateral decubitus position (i.e., right side down) taking special care to avoid excessive pressure at potential pressure points (Figure 1). The table was maintained in a neutral position, with no extension. True lateral position was confirmed by both anterior-posterior (AP) and lateral fluoroscopy. The patient was secured in position with lateral supports posterior to the buttocks and scapula and adhesive tape at the upper chest and trochanteric levels. The surface projections of the target disc space, anterior vertebral line, segmental lordotic angle for each level, and anterior superior iliac spine (ASIS) were then marked using fluoroscopy (Figure 2).
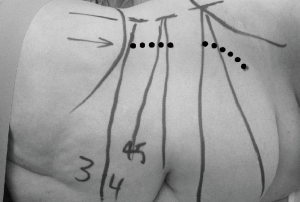
At the L3/4 and L4/5 disc levels, a single left upper skin incision oriented parallel to and 2–3 cm from the anterior vertebral line was made to expose both discs. Care was taken to ensure that the incision did not encroach on the ASIS. The L5/S1 disc was approached through a separate left oblique lower skin incision parallel to and above the inguinal ligament because of the lordotic angle of the disc space. At the L5/S1 level, the external oblique fibres are aponeurotic rather than muscular. With the L5/S1 incision, care was taken to ensure that the incision remained superior to the inguinal ligament. For multilevel lateral ALIF, the L5/S1 level was exposed first.
Dissection and instrumentation
A muscle-splitting, mini-open, retroperitoneal approach passing anterior to the psoas muscle was used to access the target disc level(s) and associated retroperitoneal vascular structures; this enabled direct line-of-sight access into the disc space. As the retroperitoneal plane was entered and carefully developed, gravity helped retract the peritoneal envelope away from the area. The abdominal wall fat, peritoneal envelope, and left ureter were retracted anteromedially to the patient’s right, thereby opening the plane between these structures and the aorto-iliac arteries, iliocaval vessels, and target disc space(s) situated posterolaterally. For multilevel ALIF, the retroperitoneal dissection used to expose the L5/S1 disc was extended superiorly to L4/5 and L3/4 as required. Prevertebral dissection and exposure of the upper disc levels then occurred via the separate skin incision, providing line-of-sight access.
Standard anterior abdominal retraction systems were used when the blade lengths were adequate. For morbidly obese (BMI >35 kg/m2) patients, the lengths of the standard retractor blades and instruments were inadequate because of the substantial operating depth. Specialized retraction systems with longer blades up to 22 cm in length (Curvy system, Relax Retractors, Sydney) were utilized for this patient group. These longer blades had bone fixation screws to improve stability and provide protection of blood vessels. Laparoscopic instruments, including a clipper, knot pusher, needle holder, and cottonoids, were beneficial.
Approach at L3/4 and L4/5
The anterior-to-psoas corridor between the psoas muscle and aorta at L3/4 and between the psoas and left common iliac vessels at L4/5 (6) was developed to access the prevertebral plane. Standard individual retractor blades were secured to the surgical drapes using Foley catheters (Figure 3). Longer blades were anchored to the vertebrae, with fixation pins placed close to the vertebral endplates to avoid the lumbar segmental vessels. A single fixation pin was placed in the L4 vertebra and utilized for exposure of both the L3/4 and L4/5 disc levels. At each level, the lumbar segmental arteries and veins were ligated and divided. At the L4/5 level, the left ascending lumbar or iliolumbar vein(s) (7,8) were likewise ligated and divided if their insertion was higher than usual. Care was taken to preserve and mobilize the sympathetic chain, which was located medial to the psoas muscle and in contact with the vertebral bodies.

Anterolateral approach at L5/S1
The prevertebral plane was approached medial to and below the left common iliac artery and vein. Optimal retroperitoneal dissection required crossing the midline. The median sacral vessels were ligated and divided. The tip of the medial retractor blade crossed the midline to rest against and pivot on the right lateral border of the target disc, which was used as the fulcrum when retracting the peritoneal envelope and left ureter. Separate inferolateral and superolateral retractor blades were placed. The left common iliac vessels (particularly the vein) were then mobilized and retracted superiorly and to the left.
Discectomy
Direct visualization of the target disc enabled annulotomy, followed by insertion of Cobb elevators and disc space distractors for discectomy and endplate preparation. Care was taken to avoid inadequate ipsilateral disc removal and endplate clearance using down-angled pituitary rongeurs, curettes, and rasps; this helped ensure midline cage positioning.
Interbody cage insertion
Integrated plate-cages (Independence®, Globus Medical, Inc., Audubon, PA, USA) or separate impacted polyetheretherketone (PEEK) ALIF cages (Perimeter®, Medtronic, Inc., Memphis, TN, USA) plus anterior titanium buttress plates (Pyramid®, Medtronic, Inc.) (Figure 4) were used, depending on the patient’s anatomy. The standalone cages were loaded using the anterolateral attachment hole to the inserter. They were initially inserted obliquely from the left side (at an angle of approximately 30 degrees); midway through insertion, the inserter was rotated laterally to orient the cage to the midline. The integrated plate-cages were loaded straight, impacted obliquely from the left side, and then midway through insertion, the inserter was rotated medially to orient the cage to the midline. All cages were filled with recombinant human bone morphogenetic protein applied to an Absorbable Collagen Sponge (ACS) (Infuse®, Medtronic, Inc.) (9).
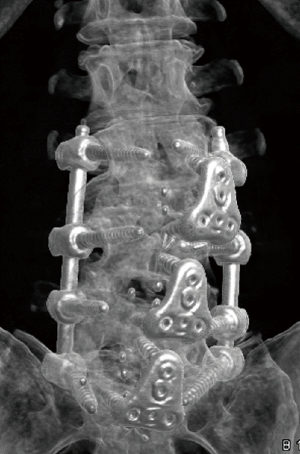
Supplemental posterior fixation
Indications for supplemental posterior pedicle screw-rod fixation followed a treatment algorithm developed by the authors (10), which included the presence of coronal/sagittal imbalance, facet arthropathy, reduced bone density, a pars defect, and pathology at >2 levels. All patients undergoing posterior instrumentation received bilateral percutaneous pedicle screws.
Clinical outcome measures
Patient-reported outcomes included the following: back and leg pain, assessed using visual analogue scale (VAS) scores; disability, assessed using the Oswestry Disability Index (ODI); and quality of life, assessed using the 36-Item Short-Form Survey (SF-36) physical component scores (PCS) and mental component scores (MCS). These outcomes were evaluated by considering the minimum clinically important difference (MCID) (11). These pre-specified MCID thresholds were used to define clinical benefit: 1.2-point improvement in back pain, 1.6-point improvement in leg pain, 12.8-point improvement in ODI, and 4.9-point improvement in PCS or MCS.
Radiological outcomes
High-definition CT scans (Somatom Definition Flash, Siemens AG, Erlangen, Germany) of the operative levels were performed preoperatively; 2 days after surgery to evaluate the instrumentation; and 6, 12, and 24 months after surgery until solid interbody fusion was confirmed on coronal and sagittal views. The postoperative scans were restricted to the operative levels, as opposed to full lumbar CT studies. To limit radiation exposure, no further scans were performed once solid interbody fusion was documented (12). The presence of bridging interbody trabecular bone indicated fusion (13). All CT scan images were interpreted by a radiologist who was not involved in the study.
Statistical analysis
The data were analysed using paired t-tests. Statistical analyses were performed using Microsoft Excel (Microsoft Office 2010, Redmond, WA, USA). Statistical significance was set at P<0.05.
Results
Patient demographics and treatment
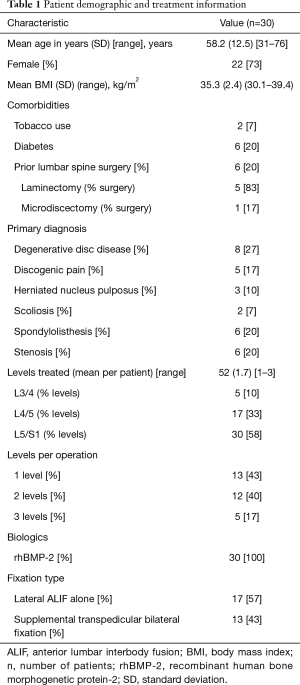
Of the 30 included patients, 22 (73%) were women. The cohort’s mean age at the time of surgery was 58.2 years (range, 31–76 years). Their mean preoperative BMI was 35.3 kg/m2 (30.1–39.4 kg/m2), which was similar to the mean BMI (33 kg/m2; range, 28–43 kg/m2) at last postoperative follow-up (P=0.83). All patients underwent L5/S1 fusion: single level at L5/S1 in 13 patients (43%), two-level at L4/5 and L5/S1 in 12 patients (40%), and three-level at L3/4, L4/5, and L5/S1 in 5 patients (17%). Mean intraoperative estimated blood loss (EBL) was 103 mL (range, 10–400 mL). Mean follow-up was 35.0 months (range, 24–48 months). Table 1 summarizes the patient demographic and treatment information.
Full table
Clinical outcomes
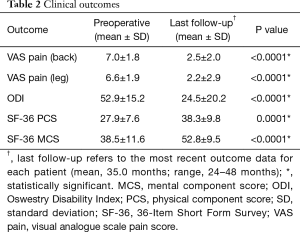
From preoperatively to last follow-up, mean back and leg VAS pain scores improved from 7.0 to 2.5 and from 6.6 to 2.2, representing improvements of 64% and 67%, respectively (Table 2). ODI improved from 52.9 to 24.5 (54%), PCS improved from 27.9 to 38.3 (37%), and MCS improved from 38.5 to 52.8 (37%). All clinical outcomes exhibited statistically significant improvement from baseline to last follow-up (P<0.05). Using MCID criteria, clinical benefit was achieved in 83% of patients for back pain, 77% of patients for leg pain, 73% of patients for disability, and 73% of patients for PCS.
Full table
Return to work
Twelve patients were retired, and 18 were working prior to surgery (12 had private health insurance and 6 were receiving Workers Compensation benefits). Of the 18, 11 (61%) resumed their preoperative employment, 3 (17%) returned to alternative employment, and 4 (22%; 2 with private insurance and 2 receiving Workers Compensation benefits) had not returned to work by the time of their last follow-up appointment. The mean return-to-work time was 5.4 months (range, 2 weeks–12 months).
Postoperative opioid use
Patients ceased opioid analgesics at a mean of 12.6 weeks (range, 2 days–6 months) postoperatively. Five (17%) of the 30 patients (3 with insurance private insurance and 2 receiving Workers Compensation benefits) were still using opioid analgesics at last follow-up.
Complications
Seven (23%) patients developed postoperative complications: anterior thigh dysesthesia (2 patients), retroperitoneal hematoma (2 patients), radiculopathy (1 patient), and subsidence (2 patients) (Table 3). Both cases of dysesthesia resolved by 6 months postoperatively. Both hematomas were managed conservatively. One patient with a persistent motor radiculopathy required second-stage posterior direct decompression surgery. One patient with a separate cage plus plate construct for multilevel (L4/5 and L5/S1) fusion developed symptomatic subsidence at L4/5; this required second-stage posterior instrumented fusion. Another patient with a single level (L5/S1) separate cage plus plate construct developed asymptomatic subsidence. No significant vascular injuries (defined as injury to a single major vessel resulting in EBL >150 mL), sympathetic trunk palsies, or retrograde ejaculation complications occurred.

Full table
Radiological outcomes
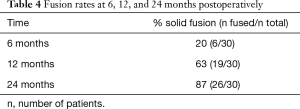
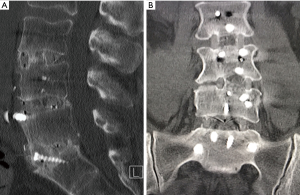
Rates for interbody fusion rates increased from 20% at 6 months to 63% at 12 months and 87% at 24 months (Table 4). An example of solid interbody fusion is demonstrated in Figure 5.
Full table
Discussion
In this study, we report our early experience with the lateral ALIF technique, which enabled L5/S1 anterior fusions to be performed in obese patients and combined multilevel fusions (at L5/S1 and L4/5, with or without L3/4) to be performed without repositioning. Lateral ALIF differs from oblique LIF (OLIF) in that it utilizes standard ALIF instrumentation, interbody cages, and plates when treating L3–S1 pathology through two incisions.
Our obese patients undergoing lateral ALIF exhibited comparable clinical outcome improvements in back VAS (64%), leg VAS (67%), ODI (54%), and PCS (37%) to those reported for our non-obese (BMI <30 kg/m2) patients undergoing supine ALIF (back VAS 57%, leg VAS 62%, ODI 54%, and PCS 42%) (14,15). Similarly, there was no difference in clinical outcomes between obese (BMI 30–34 kg/m2) and severely obese (BMI 35–39 kg/m2) lateral ALIF patients. Despite a lack of significant weight loss (BMI reduction) postoperatively, over 80% of our lateral ALIF patients achieved clinical benefit using MCID criteria for reduced back pain, and over 70% met MCID criteria for improved leg pain, disability, and quality of life. These findings suggest that primary lateral ALIF can achieve satisfactory postoperative outcomes in obese patients requiring spine surgery, without the need for major preoperative weight-loss strategies, including bariatric surgery (16). Similar improvements in function has been observed in obese patients following total hip and knee arthroplasty, without a reduction in BMI (17), and the majority of patients gain weight after major lower limb joint surgery (18,19). By 12 months postoperatively, 61% of our patients had returned to their preoperative employment. Similar return-to-work rates at 12 months were found by Singh et al. (20) in obese patients undergoing less invasive PLIF. Despite our good results, performing LIF surgery is generally accompanied by more complications in obese patients than in non-obese patients (21,22). In a retrospective analysis of 801 patients undergoing elective spinal fusion at a large institution in the United States, obese patients had a more than 2.5 times higher rate of wound and major medical complications (23).
Our lateral ALIF cohort experienced a 23% overall complication rate, similar to the 19% complication rate in our supine ALIF series (14,15). However, 2/23 patients undergoing lateral ALIF had cage subsidence, whereas none of our 131 supine ALIF patients experienced this. There were no cases of ureter or bowel injuries in either cohort, with a single case of retrograde ejaculation in our supine ALIF patients. This was lower than the 48% complication rate reported by Abe et al. in a multicentre study of 155 patients undergoing OLIF (24). The most common complications reported by those authors were subsidence or endplate fracture (19%), transient thigh pain or numbness or psoas muscle weakness (14%), and segmental artery injury (3%). In a single centre study of 137 patients undergoing OLIF, Woods et al. (25) reported a lower overall complication rate of 11.7%, with subsidence (4.4%), vascular injury (2.9%), and postoperative ileus (2.9%) being the most common complications. No vascular injuries occurred in any of our patients undergoing lateral or supine ALIF, all of which were performed in combination with a vascular surgeon. In our practice, the spine surgeon and both vascular surgeons have been working together for over 8 years and had considerable supine ALIF experience (15) prior to undertaking lateral ALIF.
Neurological complications occurred in only 3/23 patients in the present study; two patients had transient anterior thigh dysesthesia and one patient had a motor radiculopathy requiring direct posterior decompression. Neurological deficits were absent in our 131 patients who underwent supine ALIF (15) and also rare in large OLIF series reported by Mehren et al. (26) and Woods et al. (25). Similar to supine ALIF and OLIF, neural monitoring does not appear to be required for lateral ALIF.
Fusion rates at 2 years were lower in our obese patients undergoing lateral ALIF (87%) than our previously reported rates in non-obese patients undergoing ALIF (96.5–100%) or LLIF (95%) (14,15). Woods et al. reported successful CT-confirmed fusion in 95% of patients at 6 months after OLIF; however, they did not indicate the patients’ BMI (25). McAnany et al. (27) reported lower fusion rates (determined by CT) at 2 years after minimally invasive TLIF, but their rates were similar for obese and non-obese patients (45% vs. 47%, respectively).
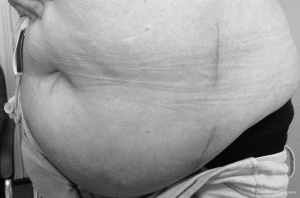
Lateral ALIF has certain benefits over both supine ALIF and OLIF. Lateral ALIF enables L5/S1 ALIF to be performed in obese patients. The approach also has procedural efficiency, permitting multilevel fusions (L3–S1) without the need for patient repositioning. In our experience, this lateral, mini-open, muscle-splitting approach is also less painful and cosmetically superior (Figure 6) to the supine, anterior midline approach for multilevel ALIF. This leads to earlier mobilization of obese patients, which may reduce postoperative morbidity. Lateral ALIF also enables direct visualization of neural, visceral, and vascular anatomy; control of potential bleeding; and repair of injured vessels, if necessary. Lateral ALIF does not utilize initial blind dilator docking, k-wires, or sequential tubular retractors. Standard anterior abdominal retraction systems can be used if the blade lengths are adequate; it not, then specialized retraction systems incorporating longer blades and bone fixation screws are used to improve stability and provide protection of vessels. An ALIF cage with a separate anterior buttress plate and an integrated plate with screws have similar biomechanical strength in all loading directions (28) and are superior in flexion and extension compared with an OLIF lateral cage with plate (29). Additionally, lateral ALIF can be combined with LLIF for higher lumbar levels (L1–L3) because both utilize the same lateral patient position. This was unnecessary in our study cohort, as no patient had disc pathology at L2/3 necessitating surgery. Meticulous use of AP and lateral fluoroscopy ensures midline positioning of interbody cages during both lateral ALIF and LLIF. Optimal cage and instrumentation positioning may be facilitated by image guidance and robotics (30).
The main limitation of this study was the relatively low number of patients undergoing lateral ALIF, reflecting the infancy of this procedure. The strengths include its prospective method of data collection and the enrolment of consecutive patients. Radiological follow-up using CT images that were reviewed by an independent radiologist increased the accuracy of our long-term fusion results. Additionally, a consistent surgical technique was used by limiting the operators to two vascular surgeons and a single spine surgeon.
Conclusions
ALIF in a lateral decubitus position enables L5/S1 anterior fusion in obese patients and permits multilevel fusions using a single patient position. Before embarking on this technique, we recommend that surgeons gain experience with lateral and anterior surgery. Satisfactory clinical outcomes and complication rates are achieved despite no reduction in BMI and 87% radiological fusion rates. Accordingly, lateral ALIF appears to be a reasonable alternative to posterior, lateral, and anterior approaches for L3/4, L4/5, and L5/S1 interbody fusions.
Acknowledgments
None.
Footnote
Conflicts of Interest: No study-specific conflicts of interest exist with any of the authors. GM Malham is a consultant for Globus, NuVasive, and Stryker; TP Wagner has received travel support from Medtronic and Synthes; and MH Claydon has received travel support from Medtronic and Synthes.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institutional ethics approval was obtained from the Epworth Hospital Research and Development Department (No. 2017).
References
- Australian Bureau of Statistics. 4338.0 – Profiles of Health, Australia, 2011–13. Overweight and obesity; 2013 June 7. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4338.0~2011-13~Main%20Features~Overweight%20and%20obesity~10007
- De la Garza-Ramos R, Bydon M, Abt NB, et al. The impact of obesity on short- and long-term outcomes after lumbar fusion. Spine 2015;40:56-61. [Crossref] [PubMed]
- Garg J, Woo K, Hirsch J, et al. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg 2010;51:946-50. [Crossref] [PubMed]
- Lee LA. Perioperative visual loss and anesthetic management. Curr Opin Anaesthesiol 2013;26:375-81. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Davis TT, Hynes RA, Fung DA, et al. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position: an anatomic study. J Neurosurg Spine 2014;21:785-93. [Crossref] [PubMed]
- Jasani V, Jaffray D. The anatomy of the iliolumbar vein. A cadaver study. J Bone Joint Surg Br 2002;84:1046-9. [Crossref] [PubMed]
- Unruh KP, Camp CL, Zietlow SP, et al. Anatomical variations of the iliolumbar vein with application to the anterior retroperitoneal approach to the lumbar spine: a cadaver study. Clin Anat 2008;21:666-73. [Crossref] [PubMed]
- Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976) 2000;25:376-81. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Maintenance of segmental lordosis and disk height in stand-alone and instrumented extreme lateral interbody fusion (XLIF). Clin Spine Surg 2017;30:E90-8. [Crossref] [PubMed]
- Copay AG, Glassman SD, Subach BR, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study Questionnaire Short Form-36, and pain scales. Spine J 2008;8:968-74. [Crossref] [PubMed]
- Richards PJ, George J, Metelko M, et al. Spine computed tomography doses and cancer induction. Spine (Phila Pa 1976) 2010;35:430-3. [Crossref] [PubMed]
- Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol 2005;26:2057-66. [PubMed]
- Malham GM, Parker RM, Blecher CM, et al. Choice of approach does not affect clinical and radiological outcomes: a comparative cohort of patients having anterior lumbar interbody fusion and patients having lateral lumbar interbody fusion at 24 months. Global Spine J 2016;6:472-81. [Crossref] [PubMed]
- Malham GM, Parker RM, Ellis NJ, et al. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine 2014;21:851-60. [Crossref] [PubMed]
- Epstein NE. More risks and complications for elective spine surgery in morbidly obese patients. Surg Neurol Int 2017;8:66. [Crossref] [PubMed]
- Donovan J, Dingwall I, McChesney S. Weight change 1 year following total knee or hip arthroplasty. ANZ J Surg 2006;76:222-5. [Crossref] [PubMed]
- Abu-Rajab RB, Findlay H, Young D, et al. Weight changes following lower limb arthroplasty: a prospective observational study. Scott Med J 2009;54:26-8. [Crossref] [PubMed]
- Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage 2010;18:510-4. [Crossref] [PubMed]
- Singh AK, Ramappa M, Bhatia CK, et al. Less invasive posterior lumbar interbody fusion and obesity: clinical outcomes and return to work. Spine (Phila Pa 1976) 2010;35:2116-20. [Crossref] [PubMed]
- Djurasovic M, Bratcher KR, Glassman SD, et al. The effect of obesity on clinical outcomes after lumbar fusion. Spine 2008;33:1789-92. [Crossref] [PubMed]
- Patel N, Bagan B, Vadera S, et al. Obesity and spine surgery: relation to perioperative complications. J Neurosurg Spine 2007;6:291-7. [Crossref] [PubMed]
- Higgins DM, Mallory GW, Planchard RF, et al. Understanding the impact of obesity on short-term outcomes and in-hospital costs after instrumented spinal fusion. Neurosurgery 2016;78:127-32. [Crossref] [PubMed]
- Abe K, Orita S, Mannoji C, et al. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery: perspectives and indications from a retrospective, multicenter survey. Spine (Phila Pa 1976) 2017;42:55-62. [Crossref] [PubMed]
- Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J 2017;17:545-53. [Crossref] [PubMed]
- Mehren C, Mayer HM, Zandanell C, et al. The Oblique Anterolateral Approach to the Lumbar Spine Provides Access to the Lumbar Spine With Few Early Complications. Clin Orthop Relat Res 2016;474:2020-7. [Crossref] [PubMed]
- McAnany SJ, Overley SC, Andelman S, et al. The effect of obesity on patient-reported outcome measures after minimally invasive trans foraminal lumbar interbody fusion. Integr Obesity Diabetes 2015;1:146-50. [Crossref]
- Schleicher P, Gerlach R, Schär B, et al. Biomechanical comparison of two different concepts for stand alone anterior lumbar interbody fusion. Eur Spine J 2008;17:1757-65. [Crossref] [PubMed]
- Fogel GR, Parikh RD, Ryu SI, et al. Biomechanics of lateral lumbar interbody fusion constructs with lateral and posterior plate fixation: laboratory investigation. J Neurosurg Spine 2014;20:291-7. [Crossref] [PubMed]
- Kochanski RB, Lombardi JM, Laratta JL, et al. Image-Guided Navigation and Robotics in Spine Surgery. Neurosurgery 2019;84:1179-89. [Crossref] [PubMed]


