
Outcomes with transforaminal endoscopic versus percutaneous laser decompression for contained lumbar herniated disc: a survival analysis of treatment benefit
Introduction
Endoscopic decompression of contained lumbar herniated disc causing sciatica-type low back and leg pain has become mainstream in many countries as an alternative to open, or other types of minimally invasive translaminar surgeries (1-4). The endoscopic surgery is typically done in an outpatient surgery center and can be done under local anesthesia and sedation (5). Therefore, it caters to the well-informed patient that is accustomed to using all sources of information at his or her disposal, including the internet, social media, and other online sources. However, for some patients with medical comorbidities, even the endoscopic surgery may be considered too aggressive of an option as they debate the risks versus reward. The thought of having surgery is associated with fear of having a surgical complication or postoperative infection, which could result in prolonged unforeseen aftercare.
Percutaneous laser decompression has seen a renaissance after nearly 20 years of relative silence and seemingly never has lost its appeal to patients (6). Most of them readily recognize and identify the laser with cutting edge technology, modern medicine, and frequently search out practices that advertise the use of lasers in their patient care programs. Newer, more advanced laser technologies and more user-friendly clinical applications with tabletop units have simplified the reintegration of lasers into an outpatient spinal surgery or office-based pain management program (7). For these reasons, percutaneous laser decompression has made a reappearance into the world of interventional pain management and is being pushed by industry to compete with other forms of minimally invasive spinal surgery including the outpatient endoscopic lumbar decompression surgery.
In this study, the authors attempted to compare clinical outcomes between visualized endoscopic transforaminal surgical and percutaneous interventional laser decompression for a defined clinical indication of contained lumbar herniated disc, causing discogenic pain and stenosis-related sciatica symptoms. This type of herniation is more common in the elderly than extruded disc herniations. In combination with age-related degenerative changes of the lumbar motion segment causing bony lateral recess stenosis, this may gradually impact the patient’s functioning and cause pain and claudication with associated disability. Typically, limited walking endurance is the consequence. The symptoms may go along with dysesthesias but are rarely associated with weakness and severe motor dysfunction. Therefore, many patients go on for years with ongoing acute on chronic episodes before they decide for interventions. The authors of this study were interested in analyzing the clinical outcomes with the transforaminal directly visualized endoscopic surgical and the non-visualized percutaneous laser interventional disc decompression by performing Kaplan-Meier survival analysis of the duration of the treatment benefit to define the clinical role of these two treatments better.
Methods
Patients
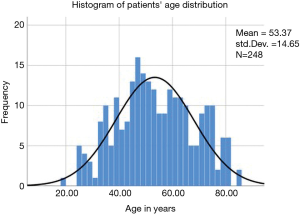
All patients in this case series suffered from sciatica-type low back and leg pain along claudication symptoms due to a contained lumbar disc herniation or lateral recess compromise. This retrospective study selected from groups of consecutive patients seen in clinics of the participating study sites. The study population was divided into two groups: Group 1 consisted of patients who underwent outpatient surgical directly visualized lumbar endoscopic transforaminal endoscopic discectomy. Group 2 patients were treated with a non-visualized interventional percutaneous laser decompression as an alternative procedure for the same clinical indication. All patients provided informed consent. The total study population consisted of 248 patients with 162 patients in group 1 and 86 patients in group 2, respectively. Patients were matched to age, gender, and diagnosis to avoid introduction of additional confounding factors or unforeseen biases. Patient enrollment at the study sites took place between 2012 and 2018. The mean follow-up was 43.5 months. The serial time recorded for Kaplan-Meier analysis ranged from 1.5 to 84 months. The patients’ age ranged from 19 to 84 years with a mean age of 53.37 years [standard deviation (SDV) =14.65 years] with a nearly normal distribution (Figure 1). There were 134 females (54%) and 114 males (46%) in the study population. The inclusion and exclusion criteria for this study have been published elsewhere in detail and are briefly described in the following (8-10).
Inclusion/exclusion and radiographic criteria
Patients were stratified for treatment by obtaining a thorough history, physical examination, and by a thorough evaluation of the advanced preoperative imaging studies. Patients with a demonstrably contained lumbar disc herniation and failed non-operative treatment for a minimum of 12 weeks were selected for this focused comparative study between the interventional percutaneous laser and the surgical and directly visualized endoscopic decompression. The size and location of the contained herniation in the spinal canal, lateral recess, and neuroforamen was graded and recorded according to well-established radiographic classification systems (11-13), which had been used by the first and senior contributing author in similar clinical outcome studies (5,8-10). Additional established radiographic stenosis parameters to assess the posterior intervertebral disc and foraminal height were employed (14). In short, a lumbar neuroforaminal height of 15 mm or less and a reduced posterior intervertebral disc height of 3 or less was graded as abnormal (14).
Moreover, reduced neuroforaminal width of 3 mm or less as measured on the sagittal MRI cuts, or lateral recess height of 3 mm or less on the axial MRI cuts were necessary prerequisites for inclusion in this study. The size of a contained central or paracentral herniation was classified as large if it measured more than 10 mm at the base on axial MRI cuts, and as small if it measured less than 10 mm. At times patients with multilevel disease underwent additional interventional work-up using a selective nerve root block protocol described elsewhere to determine the most symptomatic level best suited for intervention (15-19). For clarity of statistical data analysis in multilevel patients, the size of the herniation was recorded on the basis of the most symptomatic level. Patients with infection, tumor or metastatic disease, and spondylolisthesis were excluded. The authors considered the inclusion of rigid grade I spondylolisthesis patients but decided against it since at least in the endoscopic surgery patients (group 1) the theoretical possibility of introducing iatrogenic instability existed. Patients with severe central stenosis: <100 mm2 (20); and suspected symptoms due to facet arthropathy, or excessive facet hypertrophy were also excluded since it could impact the authors’ ability to execute the two different types of transforaminal decompression procedures.
Directly visualized transforaminal endoscopic surgical technique
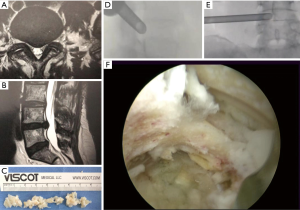
All endoscopic surgical procedures employed the transforaminal approach using the “outside-in” technique (21,22). This required the surgeons to access the neuroforamen with serial dilation and to perform a foraminoplasty using trephines and motorized drills to place a working cannula into the safe zone bordered by the pedicle inferiorly, the traversing nerve root medially, and the exiting nerve root laterally. The senior author has published the details of the surgical decompression employed in this study group elsewhere (8-10). A radiofrequency probe (Elliquence®) was used for control of bleeding, shrinkage and ablation of disc an annular tissue (23). Intraoperative fluoroscopic image guidance was used during the visualized endoscopic decompression surgery. An exemplary case is shown in Figure 2.
Non-visualized percutaneous laser decompression technique
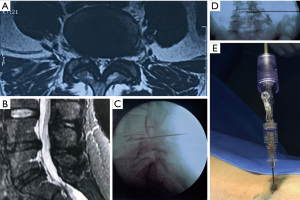
For the percutaneous laser decompression, the Leonardo® Dual diode laser platform (biolitec®) which employs two wavelengths enabling tissue interaction with both hemoglobin (980 nm) and water (1,470 nm) was used (6,7). A flexible laser fiber with a diameter of 360 µm was introduced into the center of the herniated disc via the transforaminal approach using a spinal needle (Evolve®) (7). With this technology, disc tissue is vaporized reducing the intradiscal pressure and the size of the disc herniation by thermally shrinking it (7). The resultant volumetric decompression and thermal denervation effect of nociceptive receptors in the annulus fibrosus has been credited as being the primary mechanisms of pain relief in patients with contained herniated discs (6). The biolitec® Leonardo® diode laser using a combination of 980 and 1,470 nm set at 7 W, 0.6 s pulses at 1 s intervals was used to a total energy delivered of 1,500 J. The needle placement for the percutaneous laser decompression was done with intraoperative fluoroscopic image guidance. An exemplary case is shown in Figure 3.
Clinical follow-up
The success of the visualized transforaminal or endoscopic surgical decompression or the percutaneous interventional laser procedure was evaluated using the modified Macnab criteria as the primary clinical outcome measures (24). According to Macnab, patients’ clinical outcomes were graded as Excellent if they had little pain and returned to desired activities with few limitations. Good outcomes indicated that patients had occasional pain or dysesthesias with daily activities with minor restrictions, and did not need any pain medication. If patients still needed pain medication postoperatively but still rated themselves as improved, they were assigned a Fair clinical outcome status. Patients whose function worsened postoperatively or needed additional surgery to treat residual or unresolved symptoms were categorized as having had a Poor clinical outcome from either one of the two procedures. Patients were regularly seen at 2, and 6 weeks postoperatively, and then at 3, 6, 12, and 24 months postoperatively (secular time). The time from enrollment into the study to loss of postoperative treatment benefit or deterioration (end-point variable for each patient) was recorded as the serial time which was used for the construction of survival time of the treatment probabilities and curves. Patients who maintained treatment benefit throughout the study period were censored (see below “Correlative surgical outcomes analysis”) because the total survival time for these patients could not be accurately assessed. At each follow-up visit, patients were asked whether they went to an emergency room for any unforeseen postoperative problems or whether they were admitted to a hospital for any complications or sequelae (unavoidable problems following an expertly executed surgery).
Postoperative rehabilitation
Most patients did not require postoperative rehabilitation and supportive care requirements. Some of the endoscopic decompression patients (group 1) were treated for postoperative irritation of the dorsal root ganglion with nonsteroidal anti-inflammatories, gabapentin, and transforaminal epidural steroid injections (TESI) to treat any postoperative dysesthetic leg pain syndromes.
Correlative surgical outcomes analysis
For the clinical outcome analysis, descriptive statistics (mean and standard deviation), cross tabulation statistics and measures of association were computed for two-way tables using IBM SPSS Statistics software, Version 25.0. The Pearson χ2 and the likelihood-ratio χ2 tests were used as statistical measures of association. At each individual and at final follow-up, primary clinical VAS and Macnab outcome measures were recorded and compared to their preoperative baseline. Two-tailed t-test, ANOVA testing, and cross-tabulation statistics and measures of association were computed for two-way tables using IBM SPSS Statistics software, Version 25.0. Descriptive statistic measures were used to calculate the mean, range, and standard deviation as well as percentages. Crosstabulation methods were used to assess for any statistically significant association between the type of decompression technology used and the clinical outcome data based on the modified Macnab. Pearson Chi-square and Fisher’s exact test were employed as statistical measures of association. Expected cell counts, continuity corrections, and Likelihood ratios were calculated for some analyses. Kaplan-Meier (K-M) survival time probabilities and curves were constructed from tables containing: (I) patients’ serial time; (II) their status at serial time {Macnab outcome: excellent [1], good [2], fair [3], and poor [4]; 0= censored if the total survival time for a patient could not be accurately assessed}; and (III) study group [group 1 (endoscopy) or 2 (laser)]. These tables were sorted in an ascending manner beginning with the shortest serial times for each group. Patients who were censored included patients who dropped out of the study, were lost to follow-up, or in whom required data was not available. Patients with maintained treatment benefit from either endoscopy or laser at the end of study, i.e., they survived at least until the end of the study, but there was no knowledge of what happened thereafter were also censored. The cumulative probability of surviving (continued treatment benefit with endoscopy or laser decompression) excluding censored events is seen on the Y-axis of the K-M plot allowing to analyze patient treatment intervals of varying duration. The difference between the endoscopic surgical and laser decompression survival curves was quantified for statistical significance using the log rank test which was used to calculated the Chi-square (X2) for each event time in the two treatment arms. The summed results for each group were added to derive the ultimate Chi-square to compare the full K-M curves obtained for the endoscopic surgery or percutaneous interventional laser treatment group. The confidence intervals (95%) for the Likelihood ratios were calculated using the “log method” according to Altman et al. (25).
Results
The 162 endoscopic decompression patients (65.3%) had surgery by the authors at equal portions at their respective surgical facilities. The remaining 86 percutaneous laser patients (34.7%) underwent surgery by PSTC and his team. The laterality of symptoms was nearly even with 114 patients (46%) undergoing treatment on the left, 88 patients (35.5%) on the right, and another 46 patients (18.5%) bilaterally, respectively. Paracentral disc herniations were found in 180 patients (72.6%), and central herniations were diagnosed in the remaining 68 patients (27.4%), respectively. Analysis of the MRI imaging criteria of advanced degeneration of the lumbar motion segment and the resulting stenosis in the neuroforamen and lateral recess showed the posterior disc height reduced below 3 mm in 72 patients (29%), and above 3 mm in 176 patients (71%), respectively. The lateral recess height was reduced below 3 mm in 165 patients (66.5%), and above 3 mm in 83 patients (33.5%), respectively. Large contained disc herniations (>10 mm across at their base) were noted on preoperative MRI studies of 99 of the 248 study patients (39.9%). Small disc herniations (<10 mm across at their base) were recorded in another 149 patients (60.1%).
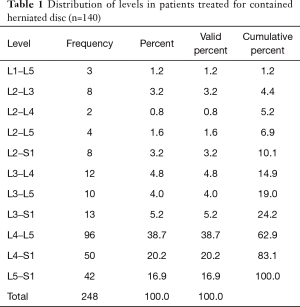
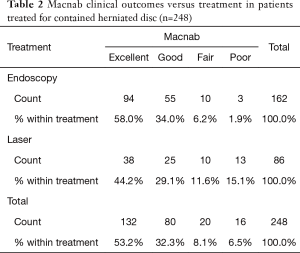
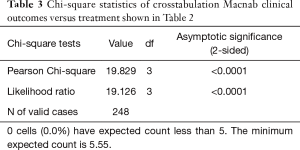
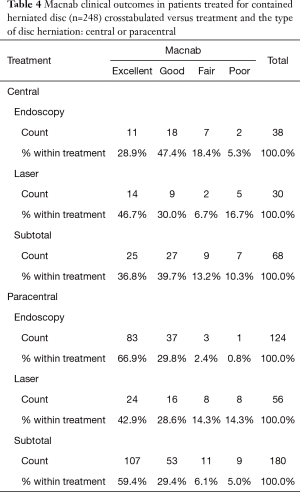
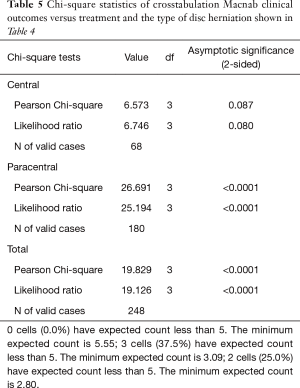
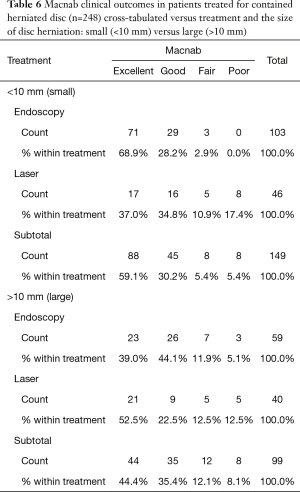
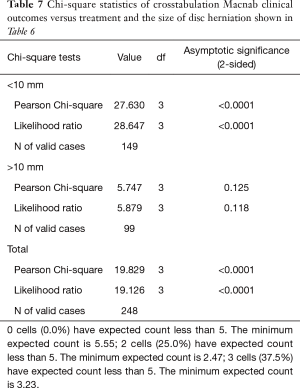
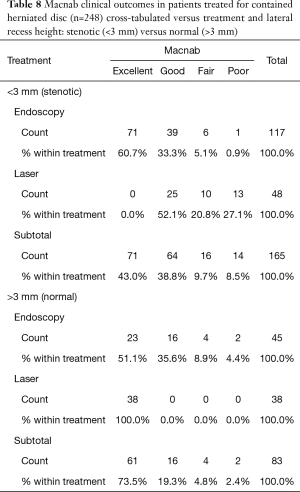
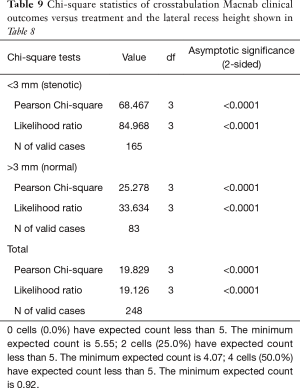
As expected, the L4/5 level was the most commonly operated level (96/248; 38.7%), followed by two-level surgery from L4 to S1 (50/248; 20.2%), and L5/S1 (42/248; 16.9%), respectively (Table 1). These levels were operated on more frequently than any other level(s) at a statistically significant level (P<0.001). There was no statistically significant difference in the level distribution between group 1 (endoscopy), and group 2 (laser) patients. However, the interventional percutaneous laser decompression was done more frequently at multiple levels. The majority of patients had excellent and good Macnab outcomes (212/248; 85.5%) regardless of treatment. Fair and poor results were achieved in another 36 patients (14.5%). Crosstabulation of clinical outcomes against the type of surgical treatment—visualized surgical endoscopy versus percutaneous non-visualized laser—showed statistically significant level of better outcomes in the Excellent Macnab category with 94 of the 162 endoscopy patients in this group (58.0%; P<0.0001). In comparison, Excellent Macnab outcomes were achieved in only 38 of the 86 patients treated with the percutaneous laser decompression (44.2%). There was also a much higher percentage of fair and poor outcomes with the laser decompression when compared to the directly visualized endoscopic surgery (26.7% laser versus 8.1% endoscopy; P<0.0001; Tables 2,3). Cross-tabulating the type of herniation and Macnab outcomes endoscopic surgery versus percutaneous laser decompression, showed a statistically significantly higher (P<0.0001) rate of excellent and good clinical outcomes with the endoscopic surgical decompression of paracentral herniations (96.8%; Tables 4,5). Further analysis of this relationship by the size of the disc herniation showed statistically significantly higher percentage of excellent and good Macnab outcomes with endoscopic decompression of small paracentral herniations (97.1%; P<0.0001). Percutaneous laser decompression of large central disc herniations was not statistically better than endoscopic surgical decompression (P=0.125; Tables 6,7). As measures of the degeneration of the lumbar motion segment, preserved posterior disc height of greater than 3 mm was associated with excellent and good Macnab outcomes in 96.7% of patients who underwent endoscopic decompression at a statistically significant level (P<0.0001). Percutaneous laser decompression in patients with advanced disc degeneration as evidenced by a posterior disc height of less than 3 mm resulted in fair and poor Macnab outcomes more frequently (47.9%) at a statistically significant level (P<0.0001; data not tabulated herein). Excellent and good Macnab outcomes were achieved in 94% of patients with lateral recess stenosis (recess height <3 mm) and were better in patients who underwent endoscopic decompression at a statistically significant level (P<0.0001). In comparison, percutaneous laser decompression for lateral recess stenosis resulted in fair and poor Macnab outcomes in the same 47.9% of patients who also had reduced posterior disc height at a statistically significant level (P<0.0001; Tables 8,9).
Full table
Full table
Full table
Full table
Full table
Full table
Full table
Full table
Full table
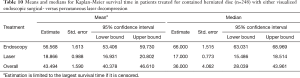
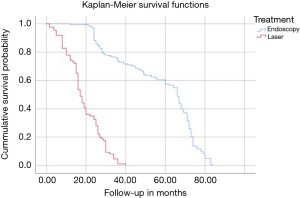
Kaplan-Meier (K-M) Survival time showed longer median survival of the treatment benefit for patients who underwent visualized endoscopic surgical decompression (66.0 months; Tables 10,11) compared to median K-M survival time for percutaneous laser decompression of 17 months (Figure 4). The log-rank test calculated the ultimate Chi-square indicating a superior median survival of treatment benefit for the visualized surgical endoscopic versus the percutaneous laser treatment at a statistically significant level (P<0.0001; Tables 10,11).
Full table

Full table
Discussion
This study aimed to analyze clinical outcomes and the duration of the clinical benefit to patients between the directly visualized transforaminal endoscopic surgery versus the non-visualized interventional percutaneous laser decompression for a contained lumbar herniated disc. The purpose of the study was simple: to delineate the role of the interventional laser decompression against the validated transforaminal endoscopic surgery. Percutaneous non-visualized laser decompression is making a comeback and is promoted as an alternative to surgery. While this may seem an appropriate course of action in patients who are either unwilling to undergo surgery or whose medical comorbidities may preclude them from it, the question remains whether these two treatments are interchangeable with equal benefit for patients. Patient dissatisfaction may arise from many areas, and anesthesia-related problems, such as postoperative nausea and vomiting are a number-one concern with patients who consider surgery (26). In this context, the authors attempted to delineate the role of both procedures better by comparing the clinical outcomes and the duration of the treatment effect by Kaplan-Meier survival analysis. The intent was to give patients more substantial clinical evidence to make the best choice of spine care in the context of their functional improvement needs at the time when the spine care is delivered.
The directly visualized transforaminal endoscopic decompression surgery techniques have been employed successfully and validated in the past (2,5,6,8-10,27). The endoscopic discectomy procedure for a herniated disc has become mainstream in most countries and is considered a viable alternative to open, and other types of minimally invasive translaminar decompression procedures. An increasing number of surgeons are using transforaminal endoscopic decompression techniques (1,5,8-10) as demonstrated and corroborated by the exponential increase PubMed and Scopus listed peer-reviewed publications in the last two years alone (27).
The situation is slightly different with the non-visualized interventional percutaneous laser decompression procedures. These have seen a surge of clinical application in the 1990s which is mirrored by a cluster of publications from that decade (28-41). As spinal endoscopy gained more traction, it became quiet around percutaneous laser decompression for herniated disc, and it is conceivable that equipment cost at the time contributed to this trend. Typical of any advanced technology, the surge of utilization was followed by a surge of complication and revision surgeries. This was no different with laser decompression where several reports of thermally damaged lumbar nerve roots appeared (42). The demise and closure of the Laser Spine Institute in the United States prompted some traditionally trained spine surgeons to announce the death of the laser in spine surgery altogether in a recent publication sponsored by the American Academy of Orthopedic Surgeons (AAOS) (43). The reality is somewhere in the middle where there are a few select centers that actively employ non-visualized laser technology for facet ablation in the treatment of mechanical back pain symptoms and the thermal shrinkage of contained disc herniations (28,44,45) and in the visualized decompression of soft tissue and bony stenosis in conjunction with spinal endoscopy technology in the treatment of sciatica-type back and leg pain (46,47).
Results of the crosstabulation and K-M survival studies showed favorable initial outcomes with both procedures in the majority of patients. However, there were some differences in outcomes when considering the type of disc herniation (central or paracentral) and its associated size. It became clear that the directly visualized transforaminal endoscopic surgery by far produced better and more durable clinical outcomes in general and in particular when compared to the non-visualized percutaneous interventional laser decompression. Clinical outcomes were statistically significantly better with endoscopic surgical than with percutaneous interventional laser decompression in patients with small paracentral disc herniations (97.1% excellent and good Macnab outcomes; P<0.0001) (Tables 4-7). Percutaneous laser decompression outcomes for large central disc herniations were statistically no better than endoscopic surgical decompression (P=0.125; Tables 6,7). Radiographic measures of advanced degeneration of the lumbar motion segment such as lateral recess height less than 3 mm were prognosticators of less favorable outcomes in general but specifically with the non-visualized percutaneous interventional laser decompression procedure (Tables 8,9) corroborating validated data in the published peer-reviewed literature that the directly visualized endoscopic bony and soft tissue decompression is more appropriate in patients with such advanced disease (47). The Kaplan-Meier (K-M) analysis of the survival times of the treatment benefit for both the endoscopic surgical and the percutaneous interventional laser decompression are starkly different and the K-M survival curves demonstrate a telling visual demonstration of the rapid deterioration of the laser treatment benefit with the 50% percentile median being 17 months. In other words, 50% of patients treated with the percutaneous laser procedure had lost the treatment benefit at 17 months postoperatively. The same K-M analysis done on endoscopic spine surgery patients revealed a median survival time of 66 months for the directly visualized endoscopic surgery (Tables 10,11).
The non-continuous nature of the K-M survival plots presented herein deserves some further discussion to avoid wrongful interpretation or extrapolation of future benefits with either of the two treatments subject to this study. The K-M survival curves provide step-wise survival estimates. They are not a smooth function. Calculating exact survival points for the endoscopic or laser decompression is actually quite difficult and depends on the number of positive and negative factors leading to the censoring of study patients with an event throughout the study or at its end without event. However, after the first patient has been censored the survival curve becomes an estimate, since it is unknown if censored endoscopy or laser patients would have experienced an event at some point later in their life. Therefore, the K-M curves show a graphic representation of the minimum survival until the first patient was censored. At that point, it is an estimation and extrapolations on future patient outcomes with these two procedures should be avoided. Since the early onset of the loss of treatment benefit with the percutaneous laser decompression (50% percentile 17 months) was in stark contrast to the long-term maintenance of treatment benefit with the surgical endoscopic decompression, this team of authors concluded that surgical treatment of sciatica symptoms due to contained lumbar herniated disc with the transforaminal endoscopic decompression was much more durable with 50% of patients maintaining treatment benefit for nearly six years.
This comparative study suffered from a few additional limitations that are worth discussing. The endoscopic surgical decompression is carried out in the epidural space with no portion of the procedure being done inside the disk space. In comparison, the percutaneous laser decompression is an intradiscal procedure in which no portion of the procedure is carried out in the epidural space. Moreover, the laser decompression is done percutaneously with the application of a small glass fiber via a spinal needle that is placed in the center of the disc for delivery of the laser energy. Therefore, no part of the laser decompression is directly visualized, and the surgeon does not obtain any additional information during the procedure that could guide him towards achieving better pain relief. In comparison, clinical outcomes with the endoscopic procedure can be maximized and improved by recognizing directly visualized painful intraoperative pathology and treating it in real time during the same surgery. Hence, one could argue that both procedures are fundamentally different and not directly comparable. From the interventional pain practitioners and patients’ point of view though, they may appear as equally effective and durable minimally invasive treatments. Marketing by industry has conditioned patients to favor laser over any surgery because of its perceived cutting-edge nature and its high-tech appearance. Similarly, lasers have been attractive for surgeons when applied during a minimally invasive endoscopic surgery due to the ability to deliver a large amount of energy through a small fiber in a very focused small area. However, the limited short-term benefit and the long-term shortfall of comparing clinical outcomes of the percutaneous interventional laser decompression with the surgical outcomes with endoscopy should be discussed with the patient.
The historical context of percutaneous laser decompression of lumbar disc herniations offers little to the understanding of the long-term survival of the treatment effect with the procedure. Most studies report short-term data. Ascher et al. deployed neodymium:yttrium-aluminum-garnet (Nd:YAG) laser through an 18-gauge needle that was introduced fluoroscopically into the intervertebral disc (34). He ablated intradiscal tissue in short bursts to avoid heating of adjacent tissues, thereby vaporizing tissue that was allowed to escape through the needle. Many subsequent authors demonstrated the utility of different types of lasers, including the Ho:YAG, which was compared to the Nd:YAG laser in a clinical trial conducted by Quigley et al. in 1991 (35). They concluded that the Ho:YAG laser was the most suitable laser at the time that they would best compromise between the efficacy of absorption and convenience of fiber-optic delivery. In 1990, Davis et al. described in 85% success rate in a study on 40 patients who underwent laser discectomy using the potassium-titanyl-phosphate (KTP 532-nm) laser (36). Only six of the 40 patients required revision with open discectomy procedures because of clinical failures. In 1995, Casper et al. described the use of the side-firing Ho:YAG laser (37) which has also been employed later by Yeung et al. (38). At one-year follow-up, Casper et al. reported an 84% success rate (37). In the same year, Siebert et al. published on 78% success rate on 100 patients with a mean follow-up of 17 months, which were treated with the Nd:YAG laser (39). Interestingly, Siebert’s study ran exactly as long as the 50% percentile median survival point in our study—17 months. Siebert’s data were corroborated by Mayer et al. who were also the first to suggest the combined use of an endoscope with laser ablation through an endoscopically introduced fiber (40). Hellinger reported in 1999 on more than 2,500 patients whom he treated with the use of the Ascher technique (34). He stated the success rate of 80% over 13 years. One year later, Yeung et al. reported an 84% success rate on more than 500 patients whom he treated with the KTP laser (38).
The current state-of-the-art of lasers in spine surgery has been summarized by Ahn et al. in a recent article (31). The authors pointed out that there are three categories of laser application in interventional and minimally invasive spinal surgery: (I) open microscopic laser surgery; (II) percutaneous endoscopic laser surgery; and (III) laser-tissue modulation for spinal pain (31). The senior author of this article has vast experience with the applications of straight- and side-firing lasers during endoscopic bony and soft tissue decompression for spinal stenosis related sciatica-type back and leg pain (38,45,46). Ahn et al. encouraged further study of the select clinical indications where efficacy has been demonstrated to substantiate the lack of evidence with randomized clinical trials (31). Brouwer et al. did provide it in their multicenter randomized prospective trial using an intent-to-treat protocol with a non-inferiority design by comparing clinical outcomes in 115 patients with sciatica-type radiculopathy due to a lumbar disc herniation occupying less than one-third of the spinal canal by randomly assigning 57 patients to conventional microdiscectomy surgery and another 55 patients to percutaneous laser disc decompression (PLDD) (28,44). The needle-based (18 G) percutaneous laser discectomy procedure afforded placement of a 600-micron glass fiber into the center of the disc similar as during a discography allowing delivery of laser energy (Diode laser, Biolitec, 980 nm, 7 W, 0.6 s pulses, interval 1 s) to a total energy delivered of 1,500 J (2,000 J for level L4–5). The authors claimed no difference in outcomes between the two treatment arms using standard clinical outcomes measures, including VAS, and Roland Morris score for back and leg pain. However, the reoperation rate in the laser group (52%) was disproportionally higher than in the microdiscectomy (21%) group (28,44). Yeung et al. reported with 8.3% a much lower comparative five-year reoperation rate with the transforaminal endoscopic lumbar decompression (45). In his study, the five-year fusion rate was only 2.7%. Although the randomized trial by Brouwer et al. did not involve application of a spinal endoscope, it provides insight into the interaction of contemporary diode lasers with typically white intervertebral disc tissue whose ability to absorb laser light in any wavelength with delivery of sufficient energy to achieve pain relief via a clinically meaningful disc decompression may be limited when placed intradiscally in a contained environment. This problem of limited energy absorption by intervertebral disc tissue during 980 nm laser decompression may genuinely be the underlying limitation of advancing the application of this technology in the treatment of intervertebral disc degeneration with or without the use of an endoscope. Any clinical trials will have to determine whether or not the in vitro demonstrated advantage in the effectiveness of the laser light treatment of bovine intervertebral disc tissue at a 1,470-nm rather than 980 nm wavelength would translate into pain relief in patients with a herniated disc (47).
The senior author has experienced the most endoscopic spine cases of over 11,000 cases over his 28-year endoscopic spine career in addition to a 20-year general orthopedic career in trauma, arthroscopic surgery, spinal deformity surgery, children’s orthopedic surgery, and hand surgery, provides him with the perspective expressed in this article. As one of the first to use and teach laser following FDA approval in 1991, he has experienced the evolution of both laser and endoscopic image-guided decompression for a broad spectrum of symptomatic degenerative and traumatic conditions in the lumbar spine. The endoscopic spinal decompression procedure correlating the pathophysiology of spinal pain with visualized pathoanatomy will continue to evolve as diagnostic methods, and surgical spine care continues to improve. In this context, the non-visualized percutaneous interventional laser disc decompression is inferior to endoscopic spine surgery. However, the last chapter in the use of laser as a dissection tool during the endoscopic decompression of spinal pathoanatomy has not been written, and more technological advancements are likely to come. What is clear is that endoscopic spine surgery and the use of lasers will likely continue to offer cost savings while providing efficacious and safe surgical spine care.
Conclusions
Transforaminal endoscopic decompression for symptomatic herniated disc is an effective and durable surgical treatment to alleviate sciatica-type and back symptoms in the vast majority of patients with good long-term survival of pain relief for up to six years. Interventional percutaneous non-visualized laser decompression for the same condition may provide favorable outcomes in the short-term. However, the treatment effect deteriorates much faster with a median survival of 17 months. Minimally invasive techniques such as the types presented in this study will continue to improve and evolve as both become more accepted and utilized worldwide.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no direct (employment, stock ownership, grants, patents), or indirect conflicts of interest (honoraria, consultancies to sponsoring organizations, mutual fund ownership, paid expert testimony). The authors are not currently affiliated with or under any consulting agreement with any vendor that the clinical research data conclusion could directly enrich. This manuscript is not meant for or intended to endorse any products or push any other agenda other than to report the associated clinical outcomes with use of endoscopy versus laser. The motive for compiling this clinically relevant information is by no means created and/or correlated to directly enrich anyone due to its publication. This publication was intended to substantiate contemporary endoscopic spinal surgery concepts to facilitate technology advancements.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. IRB approval was obtained for this study (CEIFUS 106-19). Written informed consent was obtained from the patient for publication of this Original Study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Yeung AT. Lessons Learned from 27 Years’ Experience and Focus Operating on Symptomatic Conditions of the Spine under Local Anesthesia: The Role and Future of Endoscopic Spine Surgery as a “Disruptive Technique” for Evidenced Based Medicine. J Spine 2018;7:413. [Crossref]
- Lewandrowski KU. “Outside-in” technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg 2014.8. [PubMed]
- Yeung AT, Yeung CA. Minimally invasive techniques for the management of lumbar disc herniation. Orthop Clin North Am 2007;38:363-72. [Crossref] [PubMed]
- Tsou PM, Alan Yeung C, Yeung AT. Posterolateral transforaminal selective endoscopic discectomy and thermal annuloplasty for chronic lumbar discogenic pain: a minimal access visualized intradiscal surgical procedure. Spine J 2004;4:564-73. [Crossref] [PubMed]
- Lewandrowski KU. Readmissions After Outpatient Transforaminal Decompression for Lumbar Foraminal and Lateral Recess Stenosis. Int J Spine Surg 2018;12:342-51. [Crossref] [PubMed]
- Menchetti PP, Canero G, Bini W. Percutaneous laser discectomy: experience and long term follow-up. Acta Neurochir Suppl 2011;108:117-21. [Crossref] [PubMed]
- Products. Available online: . Last accessed 06 09 2019.https://www.biolitec.com/en/products/orthopedics.html
- Lewandrowski KU. Successful outcome after outpatient transforaminal decompression for lumbar foraminal and lateral recess stenosis: The positive predictive value of diagnostic epidural steroid injection. Clin Neurol Neurosurg 2018;173:38-45. [Crossref] [PubMed]
- Lewandrowski KU. Incidence, Cost, and Management of Complications After Transforaminal Endoscopic Decompression Surgery For Lumbar Foraminal And Lateral Recess Stenosis: A Value Proposition For Outpatient Ambulatory Surgery. Int J Spine Surg 2019;13:53-67. [Crossref] [PubMed]
- Lewandrowski KU. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin Neurol Neurosurg 2019;179:74-80. [Crossref] [PubMed]
- Lee S, Lee JW, Yeom JS, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol 2010;194:1095-8. [Crossref] [PubMed]
- Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy, and surgical decompression. Spine (Phila Pa 1976) 1988;13:313-20. [Crossref] [PubMed]
- Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J 2007;16:431-7. [Crossref] [PubMed]
- Hasegawa T, An HS, Haughton VM, et al. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am 1995;77:32-8. [Crossref] [PubMed]
- Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil 2002;81:898-905. [Crossref] [PubMed]
- el-Khoury GY, Ehara S, Weinstein JN, et al. Epidural steroid injection: a procedure ideally performed with fluoroscopic control. Radiology 1988;168:554-7. [Crossref] [PubMed]
- Bogduk N, Aprill C, Derby R. Epidural spinal injections. In: White AH, Schollerman J. editors. Spinal Care: Diagnosis and Treatment. Mosby, 1995:322-43.
- Huskisson EC, Jones J, Scott PJ. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol Rehabil 1976;15:185-7. [Crossref] [PubMed]
- Lee IS, Kim SH, Lee JW, et al. Comparison of the temporary diagnostic relief of transforaminal epidural steroid injection approaches: conventional versus posterolateral technique. AJNR Am J Neuroradiol 2007;28:204-8. [PubMed]
- Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am 2003;34:281-95. [Crossref] [PubMed]
- Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976) 2006;31:E890-7. [Crossref] [PubMed]
- Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol 2005;17:641-61. [Crossref] [PubMed]
- Beyaz SG, İnanmaz ME, Zengin EŞ, et al. Combined Use of High Radiofrequency Disk Ablation, Annulus Modulation, and Manual Nucleotomy in a Patient with Extruded Disk Herniation. Pain Pract 2016;16:E74-80. [Crossref] [PubMed]
- Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am 1971;53:891-903. [Crossref] [PubMed]
- Altman DG, Machin D, Bryant TN, et al. editors. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed. BMJ Books. 2000.
- Shaikh S, Chung F, Imarengiaye C, et al. Pain, nausea, vomiting and ocular complications delay discharge following ambulatory microdiscectomy. Can J Anaesth 2003;50:514-8. [Crossref] [PubMed]
- Butler AJ, Alam M, Wiley K, et al. Endoscopic Lumbar Surgery: The State of the Art in 2019. Neurospine 2019;16:15-23. [Crossref] [PubMed]
- Brouwer PA, Peul WC, Brand R, et al. Effectiveness of percutaneous laser disc decompression versus conventional open discectomy in the treatment of lumbar disc herniation; design of a prospective randomized controlled trial. BMC Musculoskelet Disord 2009;10:49. [Crossref] [PubMed]
- Brouwer PA, Brand R, van den Akker-van Marle ME, et al. Percutaneous laser disc decompression versus conventional microdiscectomy for patients with sciatica: Two-year results of a randomised controlled trial. Interv Neuroradiol 2017;23:313-24. [Crossref] [PubMed]
- Cselik Z, Aradi M, von Jako RA, et al. Impact of infrared laser light-induced ablation at different wavelengths on bovine intervertebral disc ex vivo: evaluation with magnetic resonance imaging and histology. Lasers Surg Med 2012;44:406-12. [Crossref] [PubMed]
- Ahn Y, Lee U. Use of lasers in minimally invasive spine surgery. Expert Rev Med Devices 2018;15:423-33. [Crossref] [PubMed]
- van den Akker-van Marle ME, Brouwer PA, Brand R, et al. Percutaneous laser disc decompression versus microdiscectomy for sciatica: Cost utility analysis alongside a randomized controlled trial. Interv Neuroradiol 2017;23:538-45. [Crossref] [PubMed]
- Chang MC. Sacral root injury during trans-sacral epiduroscopic laser decompression: A case report. Medicine (Baltimore) 2017;96:e8326. [Crossref] [PubMed]
- Ascher PW. Status quo and new horizons of laser therapy in neuro-surgery. Lasers Surg Med 1985;5:499-506. [Crossref] [PubMed]
- Quigley MR, Maroon JC, Shih T, et al. Laser discectomy: comparison of systems. Spine 1994;19:319-22. [Crossref] [PubMed]
- Davis JK. Percutaneous discectomy improved with KTP laser. Clin Laser Mon 1990;8:105-6. [PubMed]
- Casper GD, Hartman VL, Mullins LL. Percutaneous laser disc decompression with the holmium: YAG laser. J Clin Laser Med Surg 1995;13:195-203. [Crossref] [PubMed]
- Yeung AT. The evolution of percutaneous spinal endoscopy and discectomy: state of the art. Mt Sinai J Med 2000;67:327-32. [PubMed]
- Siebert WE. Percutaneous laser discectomy, state of the art reviews. Spine 1993;7:129-30.
- Mayer HM, Brock M, Berlien HP, et al. Percutaneous endoscopic laser discectomy (PELD). A new surgical technique for non-sequestrated lumbar discs. Acta Neurochir Suppl (Wien) 1992;54:53-8. [Crossref] [PubMed]
- Hellinger J. Technical aspects of the percutaneous cervical and lumbar laser-disc-decompression and nucleotomy. Neurol Res 1999;21:99-102. [Crossref] [PubMed]
- Kobayashi S, Uchida K, Takeno K, et al. A case of nerve root heat injury induced by percutaneous laser disc decompression performed at an outside institution: technical case report. Neurosurgery 2007;60:ONSE171-2; discussion ONSE172.
- Radcliff K, Vaccaro AR, Hilibrand A, et al. Lasers in Spine Surgery. J Am Acad Orthop Surg 2019;27:621-32. [Crossref] [PubMed]
- Brouwer PA, Brand R, van den Akker-van Marle ME, et al. Percutaneous laser disc decompression versus conventional microdiscectomy in sciatica: a randomized controlled trial. Spine J 2015;15:857-65. [Crossref] [PubMed]
- Yeung A, Roberts A, Zhu L, et al. Treatment of Soft Tissue and Bony Spinal Stenosis by a Visualized Endoscopic Transforaminal Technique Under Local Anesthesia. Neurospine 2019;16:52-62. [Crossref] [PubMed]
- Yeung AT. The Evolution and Advancement of Endoscopic Foraminal Surgery: One Surgeon's Experience Incorporating Adjunctive Techologies. SAS J 2007;1:108-17. [Crossref] [PubMed]
- Yeung A, Lewandrowski KU. Five-year clinical outcomes with endoscopic transforaminal foraminoplasty for symptomatic degenerative conditions of the lumbar spine: a comparative study of inside-out versus outside-in techniques. J Spine Surg 2020;6:S66-S83.


