
Comparative study of curative effect of spinal endoscopic surgery and anterior cervical decompression for cervical spondylotic myelopathy
Introduction
Spinal cord dysfunction caused by spinal cord degeneration reduced blood supply is defined as cervical spondylotic myelopathy (CSM). CSM has a high incidence among middle-aged and older adults over age 55 (1,2). Patients with mild clinical symptoms may be successfully treated with physical therapy, massage, intermittent soft cervical collar bracing, and non-steroidal anti-inflammatory drugs (3). For patients with severe clinical symptoms or progressive deterioration of neurological function, timely surgical treatment is recommended (4,5).
Currently, there are many surgical methods for treating CSM. Cervical anterior decompression and fusion is still the mainstream surgical treatment of CSM. The feasibility, indications, complications, and clinical efficacy of endoscopic spinal surgery for this disease remains to be further explored. In the literature, there is no sufficient evidence to prove the advantages and disadvantages of endoscopic spinal surgery over anterior cervical decompression fusion in the treatment of CSM. Therefore, this study retrospectively analyzed 46 cases of CSM who underwent surgical treatment and complete follow-up in the General Hospital of the People’s Liberation Army for the past three years. The clinical efficacy of endoscopic spinal surgery and conventional anterior cervical decompression and fusion (ACDF) for CSM was compared, and CSM was performed. The prognostic factors were analyzed.
Methods
Patients
There were 22 patients in the spinal endoscopy group, 14 males (63.6%), 8 females (36.4%) with an average age of 42.41±7.06 years. Among them, 16 patients had single-level compressive lesions, and 6 patients had two-level compressive lesions. Two patients had a history of trauma, 14 patients suffered from upper limb motor dysfunction, 15 patients displayed lower limb motor dysfunction, and another nine patients suffered from combined upper and lower limb dysfunction. There were 24 patients in the ACDF group, 18 males (75%) and 6 females (25%), ages 46.04±8.85 years. Among them, 16 patients had single-level compressive lesions, and eight patients had two-level compressive lesions. Four cases had a clear history of trauma, 13 patients had symptoms of upper limb dyskinesia, and 18 cases of lower extremity dyskinesia, respectively. Combined upper and limb dysfunction was observed in 7 patients, and another patient had urinary retention with dysuria.
Inclusion/exclusion and radiographic criteria
The preoperative workup included routine plain film X-ray, CT, and MRI studies of the cervical spine. In the spine endoscopy group, the compressive pathology was mainly constituted by different degrees of disc herniation, posterior marginal epiphyseal hyperplasia (2 patients), and ligamentum flavum hypertrophy (2 cases), and overt spinal cord degeneration (3 patients). In the ACDF group, cervical spinal stenosis was caused by disc herniations with calcification (3 patients), posterior osteophytosis stemming from the vertebral body (5 patients), ossification of the posterior longitudinal ligament (PLL) (3 patients), and overt spinal cord degeneration (5 patients).
The Inclusion criteria were: (I) preserved motor function in the limbs, sensory dysfunction, positive pathological upper motor neuron signs; (II) preoperative Japanese Orthopaedic Association (JOA) score ≤12 points, neck and shoulder pain, and upper limb pain VAS >6 points; (III) advanced imaging findings showing compressive pathology including cervical degenerative disease, spinal stenosis, spinal cord compression consistent with the correlative clinical symptoms and signs, (IV) single- or two-level cervical spinal stenosis. The exclusion criteria were (I) osseous cervical spinal stenosis, severe vertebral posterior marginal osteophyte formation, and PLL ossification; (II) congenital developmental cervical spinal stenosis; (III) giant cervical disc herniation; (IV) cervical intervertebral disc prolapse; (V) apparent cervical segmental instability and significant kyphosis.
Endoscopic surgical technique
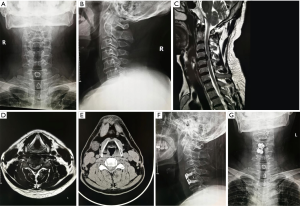
All the operations were performed under local anesthesia. For example, for endoscopic treatment of a C5/6 compressive pathology, the patient was placed in a prone position on the operating table and the neck flexed and fixed with tongues in capital flexion and cervical extension to facilitate access to the posterior elements. The C-arm was positioned over the C5/6 level in the anterior-posterior plane under fluoroscopic control. The skin entry point was marked over the surgical level, typically 1.5 cm lateral to the centerline. After standard surgical prep and layer-by-layer infiltration with local anesthesia, the 18 G spinal needle was advanced to the trailing edge of the C5 lamina. At this point, the lateral projection was checked to make sure the spinal needle used for placing the access cannula was in a good position in both planes. Them, the guidewire was placed through the spinal needle, which was then removed. A skin incision was made around both sides of the guidewire, and the subcutaneous tissues and paraspinal musculature was divided to accommodate the working cannular of the cervical endoscope—typically a round cannula with 7 mm inner working diameter. The endoscope was then used to visualize the posterior elements directly. The trailing edge of the C5 lamina was then debrided with rongeurs and a radiofrequency probe. The latter was used to ablate the remaining fibrous tissue around the surface of the lamina and the articular process to expose the V point (the lower edge of the upper lamina and the lower margin of the lower vertebrae converge on the inner edge of the facet joint). An endoscopic high-speed power burr was used to remove the lower edge of the C5 lamina and the medial edge of the facet joint, and the upper edge and lateral edge of the ligamentum flavum medially. Then, the medial aspect of the C6 superior articular process and the vertebral plate edge are decompressed to the inferior C6 lamina. Any residual obstructing bone was removed by the forceps, along with the lower edge of the ligamentum flavum as much as to expose the exiting nerve root. Intentionally, the ligamentum flavum covering the spinal cord medially was not routinely removed as the authors thought it could protect the spinal cord; particularly if additional simultaneous or staged decompressions of the contralateral lamina are contemplated. The endoscope is maneuvered left and right as well as over the top of the spinal cord into the opposite contralateral lateral cervical canal. This often requires the lower portion of the spinous process to be removed with the motorized endoscopic bur. Ultimately, the combination of these maneuvers would allow entering the opposite lateral cervical spinal canal by removing parts of the contralateral lamina similarly as on the access side. Spinal cord decompression was considered complete once the ligamentum flavum was directly visualized over the top of the spinal cord from the approach side to the opposite contralateral cervical spinal canal. When appropriate, the ligamentum flavum and the dural sac were separated with the use of a dissecting nerve hook. The intervertebral disc was decompressed by removal of material from the nucleus pulposus. Through the same skin access at an adjacent level can be decompressed if indicated. The authors have found this “single access, bilateral decompression” technique suitable for up to 3 levels. Complete hemostasis was checked before withdrawing the endoscope and working cannula. The authors prefer a single horizontal mattress stitch for skin closure. Exemplary cases are shown in Figures 1-3.
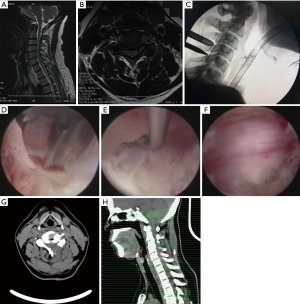
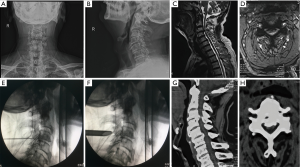
ACDF surgical technique
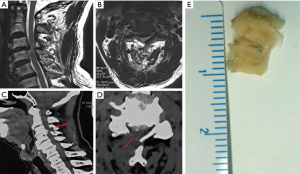
General anesthesia was used in all operations. The patients were placed in a supine position with the head in slight cervical extension. The authors preferred the right-sided Smith-Robinson approach to the anterior cervical spine. After routine surgical prep, a transverse approximately 4 cm long skin incision is made centered over the surgical level (exemplary case description for C5/6 ACDF). After standard exposure of the anterior cervical spine via division of the platysma and longitudinal dissection of the tracheoesophageal groove, Casper pins were placed into the C5 and C6 vertebral bodies to facilitate intervertebral distraction and anterior decompression of the spinal cord and the respective cervical nerve roots. First, the anterior longitudinal ligament with a sharply divided and the degenerated nucleus pulposus is removed. Posterior osteophytes or osteophytic bars are resected en bloc facilitating exposure of the posterior longitudinal ligament (PLL). The PLL was then carefully dissected off the anterior spinal cord with a nerve hook, sharply divided, and resection with Kerrison rongeurs. After decortication of the endplates and proper sizing and trailing of the interbody fusion cage, the final implant filled with autologous bone graft was placed into the C5/6 intervertebral space and fixed with an appropriately sized anterior buttress plate. Final implant position was checked on intraoperatively taken biplanar AP and lateral fluoroscopic projections. The wound was closed in layers over a small drain after copious irrigation and checked for hemostasis. A dry sterile dressing was applied, and the patient was sent to the recovery room with a soft cervical collar. Patients were instructed to remain the cervical orthosis until their first follow-up visit with their surgeon and to wear it for a minimum of 2 weeks. An exemplary case is shown in Figure 4.
Postoperative rehabilitation
In the spinal endoscopy group, patients were allowed to ambulate as early as 4 hours after surgery with their cervical soft collar in place. Postoperatively, patients were admitted to the hospital for routine intravenous infusion of mannitol and dexamethasone rehydration treatment, as well as analgesic administration for pain control and to reduce the risk of postoperative spinal cord irritation from surgical manipulation and continuous intraoperative use of irrigation fluid during the endoscopy. Patients without excessive postoperative incisional pain or any other problems or obvious complications were typically discharged to their home after a short 24-hour overnight observation stay. In the ACDF group, intravenous antibiotics were routinely administered intravenously for the first three postoperative days. The wound drain was removed on postoperative day two. Typically, patients were sent home if comfortable and without any other postoperative problems on postoperative day 4 or 5. They were sent home with their neck support and instructed to wear it for about 6–8 weeks and at a minimum to their first follow-up visit with their treating surgeon.
Follow-up and primary outcome measures
For all patients, the surgery time, intraoperative blood loss, length of hospital stay, surgery-related complications, and reoperation were recorded. Besides, the JOA scores were determined preoperatively and at three months, and one year after surgery. The JOA improvement rate was calculated at these respective follow-up times. The improvement rate = (postoperative JOA − preoperative JOA)/(17− preoperative JOA). The clinical efficacy was determined by assigning patients according to their improvement rate one year postoperatively into four grades. Improvement rates were grouped by the treating surgeon into the followed four categories according to modified Macnab criteria (6): 80% improvement rate, 50% improvement rate, 25% improvement rate and <25% improvement rate.
Statistical processing
The data were analyzed by SPSS 22.0 statistical software. The difference in primary outcomes measures between the two treatment groups was analyzed by t-test. One-way analysis of variance (ANOVA) testing was performed to calculate the percentage distribution between test groups. The data count was expressed as (%), and the comparison between groups was performed with 2-tailed t-test. The analysis of variance using repeated measures was done with a simple t-test. The impact of the JOA score changes was compared between groups and within the group. The postoperative efficacy of the two different decompression techniques was compared using the rank-sum test using a significant level of 0.05 as the acceptable P value.
Results
Twenty-two patients (47.8%) were in the spine endoscopy group and 24 patients (52.2%) in the ACDF group, respectively. Univariate analysis showed that there were no significant differences in age, gender, affected segment, clinical manifestations, and reoperation between the two groups. The data were comparable (Table 1). The average operation time, intraoperative blood loss, and length of hospital stay in the spine endoscopy group were significantly lower than those in the ACDF group, and there was a significant difference between the groups (P<0.05) (Table 2).
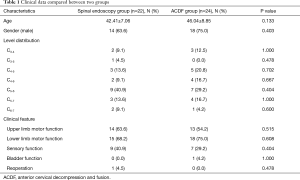
Full table

Full table
ANOVA and simple effect tests were performed on the JOA scores of the spine endoscopy group and the ACDF group preoperatively, and at three months, and one year after surgery. There were significant differences in preoperative JOA scores, three months after surgery, and one year after surgery (F=1,087.884, P<0.0001). There were significant differences between the endoscopic group and the ACDF group (F values were 492.311 and 601.219 (P<0.0001), respectively. The JOA scores in the endoscopy group and the ACDF group were significantly increased after surgery, and the symptoms gradually improved postoperatively. There was no significant difference between the endoscopy group and the ACDF group (F=0.014, P=0.905). There was no significant difference in the improvement rate of JOA scores between the two groups at three months and one year postoperatively (t=1.061, P=0.295, t=1.618, P=0.113; Table 3).

Full table
At one year postoperatively, the primary outcome measures in the spine endoscopy group were: Excellent in 11 patients (50%), Good in 7 patients (31.8%), Fair in 3 patients (13.6%), and Poor in 1 case (4.5%). Hence, Excellent and Good outcomes were achieved in 81.8% of patients in the endoscopy group. In the ACDF group, the one postoperative primary outcomes were rated as Excellent in 13 patients (54.2%), Good in 7 patients (29.2%), and Fair in 4 patients (16.7%). Excellent and Good Macnab outcomes were recorded in 83.3% of patients in the ACDF group. There was no significant difference in primary outcome measures between the two groups (Z=0.314, P=0.753; Table 4).

Full table
Discussion
This study clearly showed the feasibility of spinal cord decompression with endoscopic surgery for symptomatic spinal cord compression (CSM). The compressive pathology most commonly arises from the vertebral body and the intervertebral disc. Anterior disc herniation and the posterior spinal compression due to hypertrophy of an in folding ligamentum flavum may lead to spinal cord stenosis and ultimately produce the symptoms of florid CSM. In the authors’ opinion, decompression is the crucial part of the operation—fusion is secondary, and may be argued that fusion provides more lasting results, but inherent to the ACDF procedure as opposed to a less invasive, staged, option. Spinal endoscopy without fusion may be practical and constitute a more simplified treatment. Even for myelopathy, cervical discectomy may be sufficient to relieve myelopathy. In the endoscopic literature, investigators have primarily reported on the use of endoscopy to perform anterior spinal cord decompression procedures. The effect of primary discectomy as a long-term solution may depend on patient selection and vetting to fit the needs and activity requirement of each individual patient. There are fewer reports on the alternative posterior endoscopic resection of the lamina and the often-hypertrophied ligamentum flavum (7,8). The purpose of this study was to perform a short-term comparative analysis of clinical outcomes with the posterior endoscopic spinal cord decompression in comparison with the gold standard ACDF surgery.
The feasibility and effectiveness of excising part of the cervical lamina and the ligamentum flavum to expand the cervical spinal canal were at the heart of this study. Sven et al. (9) performed total endoscopic multi-segment laminectomy via bilateral decompression approaches on ten cadaver specimens totaling 55 segments. CT scans were performed before and after endoscopic decompression to assess the diameter of the spinal canal. Following the endoscopic decompression, the average postoperative spinal canal diameter was expanded by 4.1 mm (±1.2 mm). This cadaveric study provided proof of concept of endoscopically removing the upper part of the inferior lamina as well as the ligamentum flavum, thereby providing sufficient decompression for the cervical spinal cord. While anterior decompression and fusion of the anterior cervical spinal canal via ACDF only remove the intervertebral disc, the PLL and any osteophytes of the posterior cervical vertebral body, ACDF does not always afford the ability of adequate endoscopically visualized decompression of the posterior spinal canal space. There is no substantial volumetric expansion of the central spinal canal with ACDF (10). Posterior endoscopic decompression, on the other hand, allows for excision of bony compressive pathology or hypertrophied ligamentum flavum—both of which can lead to cervical spinal canal compromise and symptomatic CSM. The posterior enlargement of the spinal canal allows for posterior expansion of the cervical spinal cord.
Once the indication for surgery to treat CSM has been established, ACDF is currently considered the gold standard for the anterior cervical spinal cord for decompression. Its clinical efficacy has been widely recognized. The principle of the operation is to directly decompress the spinal canal by removing the intervertebral disc and any associated posterior apophyseal ring osteophytes protruding into the front of the spinal cord thereby aiding in the restoration of spinal cord function. In comparison with ACDF, the indications for endoscopic spinal surgery for CSM are relatively narrow. The authors stipulated that endoscopic treatment of patients with CSM can remove the posterior lamina and the thickened in folded ligamentum flavum, thereby, enlarging the volume of the spinal canal, and achieve the purpose of decompressing the spinal cord. However, it is relatively tricky to endoscopically treat the intervertebral disc with prominent degeneration in the front of the spinal cord. Additional anterior compressive pathology including epiphyseal osteophytes emanating from the vertebral body and a calcified PLL is also challenging to address with the posterior endoscopic decompression procedure. Even if technically feasible, forced removal of an anterior intervertebral disc protrusion may lead to damage of the spinal cord and cervical nerve roots. In cases of large disc herniation, ossification of the PLL and severe hyperplasia of the posterior vertebral body, endoscopic spinal surgery should not be used. However, for elderly CSM patients with a poor general health condition and multiple chronic comorbidities, endoscopic spinal cord decompression is an attractive alternative to traditional open spinal cord decompression particularly in the elderly who cannot tolerate traumatic open fusion surgery. The authors emphasize the need to strictly adhere to inclusion and exclusion criteria given the high-risk nature of the surgery. Therefore, a high surgical skill level is required to consistently achieve satisfactory clinical outcomes commensurate with the published data for the competing ACDF procedure. The authors of this publication recommend hands-on training in cadaver training courses and side-by-side to a mentoring master surgeon.
The comparative analysis of posterior spinal endoscopic decompression and ACDF showed significantly reduced average operation time, intraoperative blood loss, and length of hospital stay in the spinal endoscopy group than in the ACDF group. The difference was statistically significant, objectively indicating that the endoscopic surgery as a more simplified surgical treatment for CSM was equally effective at least short term as the anterior cervical decompression while providing several advantages. Avoiding fusion can significantly reduce the operation time, intraoperative blood loss, and reduce the risk of surgery and postoperative infection rate. Also, the endoscopic decompression maintains the integrity of the posterior interspinous ligament—an important anatomical tension band mechanism. Additionally, endoscopic spinal surgery has the advantages of reduced tissue trauma, reduced incision pain and, hence, rapid postoperative recovery, and short hospital stay. While in China all patients are frequently admitted to the hospital as an inpatient for some days, it is the authors’ opinion that it is technically feasible to perform the endoscopic cervical decompression surgery on an outpatient basis by sending patients home after a reasonably short time of observation. The outpatient surgery concept is supported by the low-risk nature of traversing the posterior cervical spinal anatomy. Serious peri- and postoperative complications are uncommon. The ACDF technique, on the other hand, uses the anterior cervical approach, and there are many vital anatomical structures in the neck. The possibility of serious complications may readily arise from injury to the esophagus, trachea and the neurovascular bundle in the carotid sheath. Several technique considerations are unique to the spinal endoscopy procedure, which improve visualization due to reduced bleeding, and magnification under the continuous irrigation with normal saline. Accurate and precise identification of the spinal cord and cervical nerve roots in the authors’ experience is superior to microsurgical dissection during ACDF and may reduce the risk of dural tears and nerve root injuries (10-12). Additional advantages may include a lower incidence of postoperative kyphosis and axial neck pain. Consequently, there may be a reduced need for fusion in selected patients, and reduced risk of subsequent degeneration of adjacent segments with the motion-preserving endoscopic decompression surgery.
Nevertheless, these advantages can only play in the most skillful hands. The technically demanding surgery involves the use of high-speed power drills during the removal of the upper and lower lamina, and the medial portion of the articular pillar of the cervical facet joints requires to overcome a significant learning curve. Individual learning steps should focus on first thinning out the lamina, and then removing the remaining laminar bone with endoscopic rongeurs to complete the spinal cord decompression to prevent injury to the spinal cord and nerve roots. Only the central portion of the ligamentum flavum is removed as its lateral portion can protect the spinal cord and nerve roots when the base of the spinous process and the contralateral lamina are treated with a power drill. When removing the ligamentum flavum, the ligamentum flavum is dissected off the nerve root to free it entirely and to avoid dural tears and adhesions with the spinal cord. The facet joint resection should not exceed 50% of the joint pillar. Otherwise, axial neck and shoulders pain may ensue postoperatively. Conceivably, it could prompt instability, and even compromise of the vertebral artery and other serious consequences (12).
This study has limitations, including a short follow-up time of only 12 months. Therefore, the long-term effect of endoscopic spinal treatment of CSM needs to be assessed beyond one-year follow-up. Moreover, the number of cases in this study is small, and all patients came from one practice setting at the same hospital. Hence, there may have been some selection bias in choosing patients for the endoscopic decompression procedure (3). The authors also recommend additional research on the relationship between duration and severity of preoperative symptoms and upper motor neuron signs, neurological status, postoperative symptom resolution, and overall prognosis.
Conclusions
The short and medium-term clinical outcomes and efficacy, endoscopic spinal surgery, and ACDF for the treatment of CSM in skilled hands are equivalent. Spinal endoscopic cervical spinal cord decompression is an attractive alternative for the treatment of CSM. However, the indications are narrow due to the inability to address anterior compressive pathology. Spinal endoscopic surgery has apparent advantages such as reduced operative time, intraoperative blood loss, and hospitalization. The technique warrants further clinical investigation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no direct or indirect conflicts. This manuscript is not meant for or intended to endorse any products or push any other agenda other than the associated clinical outcomes with endoscopic spine surgery. The motive for compiling this clinically relevant information is by no means created and/or correlated to directly enrich anyone due to its publication. This publication was intended to substantiate contemporary endoscopic spinal surgery concepts to facilitate technology advancements.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the First Medical Center, General Hospital of PLA. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician 2000;62:1064-70, 1073. [PubMed]
- Bernhardt M, Hynes RA, Blume HW, et al. Cervical spondylotic myelopathy. J Bone Joint Surg Am 1993;75:119-28. [Crossref] [PubMed]
- Kuijper B, Tans JT, Beelen A, et al. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomised trial. BMJ 2009;339:b3883. [Crossref] [PubMed]
- Klein GR, Vaccaro AR, Albert TJ. Health outcome assessment before and after anterior cervical discectomy and fusion for radiculopathy: a prospective analysis. Spine (Phila Pa 1976) 2000;25:801-3. [Crossref] [PubMed]
- Fang M, Lu J, Wei Y, et al. Early outcome of using Zero-profile implant system in treatment of cervical spondylosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2013;27:1206-9. [PubMed]
- Minamide A, Yoshida M, Yamada H, et al. Clinical outcomes of microendoscopic decompression surgery for cervical myelopathy. Eur Spine J 2010;19:487-93. [Crossref] [PubMed]
- Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am 1971;53:891-903. [Crossref] [PubMed]
- Yabuki S, Kikuchi S. Endoscopic surgery for cervical myelopathy due to calcification of the ligamentum flavum. J Spinal Disord Tech 2008;21:518-23. [Crossref] [PubMed]
- Eicker SO, Klingenhöfer M, Stummer W, et al. Full-endoscopic cervical arcocristectomy for the treatment of spinal stenosis: results of a cadaver study. Eur Spine J 2012;21:2487-91. [Crossref] [PubMed]
- Ruetten S. Full-endoscopic Operations of the Spine in Disk Herniations and Spinal Stenosis. Surg Technol Int 2011;21:284-98. [PubMed]
- Ruetten S, Komp M, Hahn P, et al. Decompression of lumbar lateral spinal stenosis: full-endoscopic, interlaminar technique. Oper Orthop Traumatol. 2013;25:31-46. [Crossref] [PubMed]
- Shin DA, Kim KN, Shin HC, et al. The efficacy of microendoscopic discectomy in reducing iatrogenic muscle injury. J Neurosurg Spine 2008;8:39-43. [Crossref] [PubMed]


