
Cervical laminoplasty: indication, technique, complications
Introduction
Cervical myelopathy refers to compression of the cervical spinal cord from dorsal and/or ventral lesions which in turn leads to a distinctive clinical presentation (1). When caused by degenerative or spondylotic changes, cervical spondylotic myelopathy (CSM) is most commonly seen in older individuals with loss of integrity of the intervertebral disc, facet and uncovertebral joint osteophytes as well as ligamentum flavum hypertrophy (2). Congenital narrowing of the spinal canal may exacerbate symptoms in older individuals, and may predispose younger patients to early onset CSM (3). Because initial symptoms can be subtle, a delay may be encountered before patients present to a spine care provider (4). The natural history of CSM has been well-described; most patients experience a gradual, stepwise deterioration with limited potential for spontaneous resolution, although there exists a wide clinical spectrum (5,6). Therefore, surgical treatment is often recommended as the most effective means to limit progression of symptoms.
The optimal surgical technique for addressing CSM remains controversial. One approach is not superior in every circumstance, and the best operation in any given individual will depend on anatomic and symptomatic factors specific to that patient. Anterior surgery allows direct access to ventral compressive structures (7,8). Posterior surgery facilities the decompression of multiple levels of pathology, potentially without necessitating fusion, and may be used in conjunction with anterior surgery, or alone. In addition to being able to directly decompress posterior compressive lesions, an indirect decompression of the anterior elements can also be achieved (Figure 1). This allows for canal expansion and a drift of the cord away from offending anterior pathologies (9). First described in 1973, laminoplasty has been increasingly implemented as a non-fusion, motion preserving decompression of the cervical spinal cord (10).
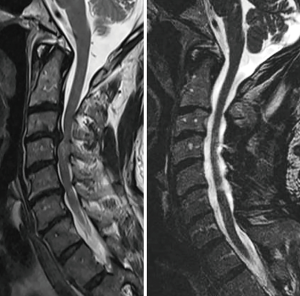
Laminoplasty offers several advantages over laminectomy with or without fusion (10-13). It is not subject to the complications associated with arthrodesis, such as potential for accelerated adjacent segment disease and pseudarthrosis. Compared with laminectomy alone, preservation of the posterior tension band allows for a more physiologic loading and better replicates native biomechanics with laminoplasty (14). Although loss of lordosis can occur with laminoplasty, it is generally not associated with the type of catastrophic kyphosis that can be seen after multilevel laminectomy alone. In addition, maintaining dorsal coverage over the dura with laminoplasty prevents the formation of the post-laminectomy membrane and allows for safer revision procedure if necessary (11). Although the amount of high-quality data comparing outcomes after laminoplasty versus laminectomy and fusion is limited, a matched cohort study found that laminoplasty may be associated with fewer complications and better clinical outcomes in appropriately selected patients (15,16).
Various laminoplasty methods have been described in the literature. In each of the described techniques, the critical aspect involves enlarging the diameter of the spinal canal through the creation of an expanded laminar arch (15,17). Stabilization of the expanded lamina is then achieved using a variety of techniques (9,12,18,19).
Indications
Laminoplasty was originally described as a non-fusion alternative to decompress multilevel spinal cord compression while avoiding post-laminectomy kyphosis (10-12). In our opinion, the ideal indication for laminoplasty is in a patient with multilevel myelopathy (generally involving 3 or more motion segments), who has preserved lordosis, and minimal to no spondylotic axial pain. It is important to keep in mind that these are the ideal indications, and that patients who do not perfectly fulfill all of these criteria may still be candidates for laminoplasty.
Since it does not involve fusion, laminoplasty is not intended to treat spondylotic axial neck pain. Those who have a primary complaint of axial pain may be better served with a fusion-based alternative. However, many patients with myelopathy have little to no axial neck pain. In a recent study, we demonstrated that, in such patients, laminoplasty does not increase neck pain scores postoperatively (20). Gross instability, particularly if it causes associated exiting root compression, may be a contraindication to laminoplasty, however, the literature shows that laminoplasty can be successfully performed in those with mild spondylolisthesis (21). Similarly, it is important to remember that the primary goal of the operation is decompression of the spinal cord, and therefore is not generally the procedure of choice in patients with pure radiculopathy who may be better managed with a foraminotomy or anterior procedure. Inflammatory arthritis such as ankylosing spondylitis (AS) and rheumatoid arthritis (RA) also serve as a relative contraindication (19), although some centers have reported acceptable outcomes in modern studies on patients receiving appropriate medical management (22).
Most studies suggest that patients can experience a moderate improvement in modified Japanese Orthopaedic Association (mJOA) scores following surgery. In a recent meta review of outcomes after CSM, Bartels et al. reviewed a series of 28 cohorts and found that postoperative mJOA score was 14.1, improved from 10.1 preoperatively. This was not significantly different from the mJOA scores of a similar cohort of laminectomy and fusion patients where mJOA scores were 13.8 postoperative (9.4 preoperatively) (23). Similarly, a prospective, randomized control trial also found no differences in JOA scores between laminoplasty and laminectomy and fusion, however, those authors reported that laminoplasty offered an improvement in Nurick grade postoperatively (24). Most studies have shown that complication rates in laminoplasty are similar, or slightly less than other decompressive procedures (23). To our knowledge, there exists no definitive data to suggest any difference in mJOA scores or any other quantitate outcome after laminoplasty, laminectomy and fusion, or anterior cervical decompression and fusion performed for CSM. Therefore, the decision to perform one operation should be based on the location of the pathology, number of levels affected, and sagittal balance, among other factors (23). We would agree with the conclusions of Klineberg et al. who recommend surgeons approach each case individually, and consider the advantages and disadvantages of each of the above mentioned procedures. Laminectomy alone (without fusion), however, is rarely indicated (25).
Sagittal balance
Laminoplasty is ideally suited to a lordotic spine because lordosis allows for greater drift-back of the spinal cord—away from any impinging anterior structures—after the posterior laminar arch has been expanded. Although laminoplasty has been associated with successful neurologic outcomes in patients with up to 13 degrees of kyphosis (26), we generally prefer patients to have lordotic, or, at a minimum, neutral sagittal alignment because some loss of lordosis, and even kyphosis, can occur after laminoplasty (27). Therefore, although there are techniques that can be employed to mitigate loss of lordosis that will be discussed below, it is advantageous to start with a lordotic reserve. Traditionally, the literature has similarly advised laminoplasty be avoided in patients without lordosis (28). Recent reports have confirmed early recommendations that laminoplasty be avoided in patients with obvious cervical kyphosis, although specific measurement parameters vary in the literature, with most authors recommending at least a neutral C2–7 lordotic angle (18,29).
In addition to cervical lordosis, both local cervical sagittal balance and even global thoracolumbar sagittal balance may affect laminoplasty. Matsuoka et al. recently evaluated 84 consecutive patients undergoing laminoplasty for CSM and found that in patients without preoperative cervical kyphosis, those with increased lumbar lordosis and a small pelvic incidence-lumbar lordosis (PI-LL) (truncal offset) were more likely to report postoperative cervical kyphosis (30). Furthermore, Kato et al. performed a retrospective review of 110 patients and found the C2–7 sagittal vertebral axis (SVA) measurement was most predictive of axial neck pain (31). Patients with a C2–7 SVA greater than 3.5 cm had higher reported pain scores, although their improvements in terms of myelopathy (JOA) were not significantly different. Conversely, Sakai et al. found that in patients with excessive forward head pitch measured by the center of gravity of the head (CGH)-C7 SVA, neurological recovery after laminoplasty was inferior to laminectomy and fusion (32).
Although the importance of cervical lordosis in laminoplasty is well established, much less is known at this point about the impact of cervical and global sagittal alignment. Based on our experience, however, we recommend consideration of both the C2–7 lordotic angle and cervical sagittal balance when contemplating laminoplasty.
Technique
Anesthesia
Extreme neck hyperextension during intubation could cause worsening spinal cord compression and should be avoided (11-13). In the vast majority of cases, a glidescope can be used to facilitate intubation without cervical extension. At the discretion of the surgeon and anesthesia team, a fiberoptic or video laryngoscope intubation may be necessary in unusual circumstances. Intraoperative hypotension should also be avoided. There is no established threshold regarding what constitutes appropriate arterial perfusion, although we routinely advocate for a mean arterial pressure (MAP) goal of greater than 80 when clinically feasible. Patients with pre-existing hypertension may require higher MAP goals to ensure sufficient blood supply to the spinal cord. Additional access lines may be required in those with cardiac lability to ensure this can be done reliably (9).
Spinal cord monitoring
Neurological monitoring is generally recommended during laminoplasty, although can be considered at the discretion of the surgeon (11-13). This affords the surgeon and anesthesiologist useful information regarding cord profusion and can detect peripheral nerve palsies secondary to positioning. Baseline potentials should be obtained in the prone position after all positioning adjustments have been made (33). We generally do not obtain pre-positioning data in laminoplasty patients.
There are well-described advantages and disadvantages to somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs), with MEPs generally being more sensitive, but less specific than SSEPs (11-13). For this reason, the senior author generally utilizes SSEPs alone during laminoplasty to avoid the potential false-positives seen with MEPs, and reserves MEPs for deformity correction surgery (34).
Patient positioning
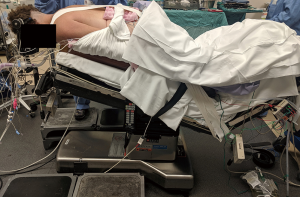
Appropriate positioning is a key to the success of the operation, and the surgical team should be actively involved from the time the patient enters the operating room (11-13). Cervical tongs are applied to the patient in a standard fashion. Gardner-Wells tongs may be considered, although it is the senior author’s preferred technique to use the Mayfield head holder as it allows easier control of flexion/extension and allows for very reliable control of the head. The patient is positioned prone, and longitudinal bolsters are placed along the bed in order to allow the abdomen to hang freely and decrease intra-abdominal venous pressure. The bed is then placed into reverse Trendelenburg to decrease venous congestion at the surgical site. The distal end of the bed is gently flexed at the patient’s knees—this prevents caudal migration of the patient during the operation. The knees are padded with foam donuts, and the shins supported with padding as well. The arms are tucked at the sides of the patient with thumbs pointed towards the floor. The shoulders are lightly taped to prevent excessive forward flexion and to move them out of the way of a lateral intraoperative X-ray (13).
The head and neck should then be inspected again, and slight modifications in the position of the Mayfield head holder can be performed at this time. A slightly flexed or neutral posture is desired, with the chin partially tucked, as this avoids the increased shingling, or overlap, of vertebrae seen in an overly extended alignment. This position also enlarges the spinal canal slightly to avoid further compression intraoperatively. Once ideally positioned, the sagittal alignment of the neck should be roughly parallel to the floor (Figure 2) (11-13).
Surgical approach
Prior to incision, the surgeon should again confirm appropriate patient positioning and communicate with the neuromonitoring and anesthesia teams that all lines and signals are running appropriately. The back of the neck and skull base is shaved, and in individuals with long hair we often tape the remaining hair proximally, retracted away from the surgical field. The head and neck are prepped and draped in standard fashion. The surgeon can feel for superficial landmarks: the posterior occiput and skull base, odontoid, and vertebrae prominins should be palpable even in obese patients.
The skin is incised sharply and a direct, midline longitudinal approach is carried down to the level of the fascia with electrocautery. Great care should be taken to stay in the midline raphe in order to minimize bleeding and muscle trauma (11-13). The raphe is easily identified by a relatively avascular white stripe spanning cranio-caudally. The dissection should then be carried down to the spinous processes in a subperiosteal fashion along the lamina. Oftentimes, it is easier to begin the deep dissection at the caudal extent of the wound as the cranial fascia is elevated from the respective spinous processes. We painstakingly preserve all of the extensor muscle insertions on to the C2 spinous process whenever possible. Distal insertions on to C7 or T1 are also preserved to the extent possible (11-13). Dissection should be continued to the lamina-lateral mass junction. Assuming plate fixation is to be used, this can be carried out to the center of the lateral masses on the “open” side (see below). Radiographs should be taken to confirm appropriate levels. At this point, the interspinous ligament should be removed at the cephalad and caudal limits of the planned construct (35).
Developing the opening and hinge troughs
The open trough should be created first (Figure 3). We generally perform this on the side with greater clinical symptoms, although either side can be utilized depending on surgeon preference. For patients with myeloradiculopathy, one may more easily add foraminotomies on this side (see below).
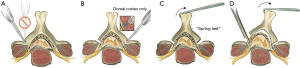
The opening trough is created with a burr at the junction of the lamina and lateral mass as would be done during a laminectomy. Various techniques have been described, although we prefer to sequentially burr each level through both the outer and inner cortices, or until only a translucent wafer of bone on the inner cortex is remaining (11-13). In addition to receiving tactile feedback from the burr, the surgeon can often observe the yellowish tint of the ligamentum flavum and the bluish tint of the dural sac through the remaining wafer of bone once the appropriate amount has been removed. The cranial aspect of the lamina is often covered by a shingling or overlap from the lamina below. This overlapping bone needs to be removed with a burr in order to gain sufficient access to the cranial lamina. It is also important to recognize that the lamina is thicker at its cranial aspect and has no ligamentum flavum covering its ventral aspect. Therefore, dural injury, although rare is at greater risk when working on the cranial versus caudal lamina. Any small remaining flake of ventral cortex can be removed with a micro-curette or Kerrison rongeur as needed (12,13).
The hinge is then created on the contralateral side. Preservation of the ventral cortex of bone on this side is important. Again, the burr is placed at the junction of the lamina and lateral mass, however it is only used to remove the dorsal cortex of bone. Since the contralateral side has already been completely opened, the hinge mechanism can be continually tested to see if adequate bone has been removed from the hinge side to allow for the opening affect to take place. This is done in succession at each level (11-13).
Opening the laminoplasty
Assuming the opening and hinge troughs have been appropriately created, the laminoplasty is opened in sequential fashion. The surgeon can use instruments to apply dorsally directed pressure underneath the lamina and manipulate the spinous process dorso-laterally such to facilitate dorsal and lateral opening at each level. As this is done, underlying ligamentum flavum will present itself on the opening side and newly introduced tension will facilitate its removal with a kerrison.
At this point, the interlaminar spaces at the ends of the construct should be inspected again, and underlying ligamentum should be resected here as well. Venous bleeding encountered during laminoplasty can be controlled with a combination of bipolar cautery and thrombin foam. Definitive hemostasis is easier to achieve once the laminoplasties have been fully opened segmental venous congestion relieved (11-13).
Internal fixation
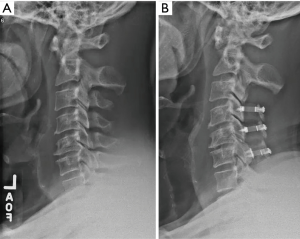
After the laminoplasty has been opened at each level, segmental fixation is applied to maintain the opening. A variety of laminoplasty plating systems are now commercially available. We generally prefer plates due to ease of application as well as the immediate, stable fixation provided (Figure 4). In a series of plate-only laminoplasty with no supplemental bone graft at 217 levels, we found maintenance of a stable expanded laminar arch in all levels, with zero plate failures, dislodgements, premature closures, or adverse neurologic consequences (36). Ninety-three percent of the hinge segments had healed by CT scan at 12 months, and even those that did not demonstrate bony union nevertheless maintained a patent, expanded, laminar arch. Depending on bone quality, one or two screws are placed into the cut edge of the lamina, and two screws into the lateral mass (37).
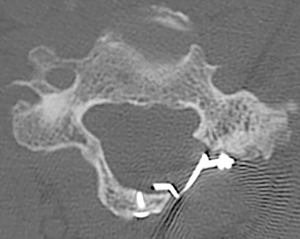
Alternative options for fixation include sutures, suture anchors, and bone. Hirabayashi et al. described a technique where sutures are places from the spinous process into the facet hinge side (38), however, longitudinal studies have shown this construct may be subject to premature closure (36). Bone struts have been used as an interspace (9,39), however failure can lead to complications such as closure or intrusion of grafts into the spinal canal (40).
Alternative technique: preservation of the muscle-ligament complex on the hinge side of open door laminoplasty
One of the criticisms of traditional open-door technique is the potential for increased axial neck pain (11-13). Yoshida et al. have described an alternative technique to mitigate this risk which involves the preservation of soft tissues on the hinge side (41). In this variation, the paraspinal muscles are dissected from the opening side as described above. An osteotomy is performed through the spinous processes and is retracted towards the hinge side. The surgeon is now able to access the contralateral lamina-lateral mass junction through the osteotomy and create the hinge as would be done in earlier techniques. Securing the extensor musculature and spinous processes back to midline is performed during closure (14,19,28,41).
Alternative technique: French door laminoplasty
The French door variant was first described in 1982 as an alternative method versus the open-door approach (42). The main technical difference lies in the creation of bilateral hinges at the lateral mass-lamina junction, and opening through the midline of the lamina. The bilateral opening has been compared to that of a French door that swings open in the center. While fundamentally similar, proponents of the French door technique will point to potentially lower blood loss (avoiding the lateral epidural veins), and a more symmetric direct decompression of the canal. Criticisms of this technique center on the need for three cuts instead of two, one of which is directly in the midline over the compressed spinal cord.
Adjunctive technique: foraminotomy
In patients with radicular symptoms, the surgeon may choose to add foraminotomy to any of the above-mentioned techniques. Although technically easier to perform on the opening side, foraminotomies can also be performed on the hinge side. When performed on the hinge side, they should be done prior to opening of the laminoplasty. When performed on the opening side, we would suggest this be done after application of internal fixation, when the canal has already been opened and there is greater access to the foramen.
Prophylactic foraminotomy has been advocated in the literature as a method of decreasing the rate of postoperative C5 palsy. However, there is conflicting data as to its efficacy in achieving that goal. As a result, we do not generally advocate performing prophylactic foraminotomies of asymptomatic levels during laminoplasty.
Alternative technique: fusion and/or hybrid fixation
When desired, the surgeon can choose to fuse certain levels and perform laminoplasty at other levels. This type of hybrid fixation can be applied at the surgeon’s discretion on a level-by-level basis.
Similarly, there exists the potential for laminoplasty and fusion at the same level (11-13). The theoretical advantage of laminoplasty over laminectomy and fusion is the larger contact area available for fusion, although this comes at the expense of less available local bone graft. If the surgeon is to choose this type of fixation construct, it is important to plan in advance, as placement of lateral mass fixation must precede opening the laminoplasty hinge (9,12).
Complications
Most data suggest that the overall rate of complication following laminoplasty is comparable, or less than other operations for myelopathy (39). As with any procedure, the surgeon should be transparent regarding expectations and appropriate disclosure of the following potential complications is advised (11-13).
Wound healing
The posterior cervical approach is generally associated with higher rates of wound complications when compared to anterior approaches (39,43,44). The use of topical vancomycin powder may decrease the number of post-operative infections (45). Despite this, rates of surgical site infection are reported to be as high as 5–8% in some studies, and sterile fluid collections are even more common (46,47). Similarly, delayed wound healing may occur, especially in patients with comorbidities, as is commonly seen in this patient demographic. We routinely use a subfascial drain, although recent studies have challenged this practice (48). In select circumstances, the surgeon may consider vacuum-assisted closure.
Proper soft tissue management is arguably more important when performing laminoplasty versus laminectomy with fusion, as functioning cervical extensor muscles are required to maintain the posterior tension band in the absence of arthrodesis. Although traditional approaches described detachment and then reattachment of the extensor muscle insertions on to C2, we now try to avoid any detachment during exposure whenever possible. The insertions on to C7 and T1 are also preserved to the extent possible, but may not be as critical. Meticulous hemostasis is obtained prior to closure. We perform a layered closure technique, first closing the cervical extensor muscle in a separate layer deep to fascia. In addition to closing dead space, this also serves to improve the cosmesis of the wound, preventing the “sunken-in” appearance that can occur due to diastasis. Care should be taken not to strangulate the muscle during closure but rather gently approximate the edges. The fascia is then closed in a watertight fashion, incorporating the muscle as well to achieve a unified layer of closure (12,13).
Loss of sagittal alignment
As discussed above, there has been greater recent interest on sagittal alignment and its effect on clinical and radiographic outcomes after laminoplasty. In general, we avoid laminoplasty in patients with kyphosis. In addition, in patients with significantly increased C2 or CGH SVA, we may consider alternatives to laminoplasty even if the spine is lordotic (30).
Some loss of lordosis can occur after laminoplasty, although it rarely leads to the type of severe kyphosis seen after multilevel laminectomy. In one series, the average loss of lordosis was 5°, with up to 11% of patients falling into net kyphosis (12,29,31,49). We have utilized the following technique to try to decrease the amount of lost lordosis after laminoplasty (Figure 5). By performing a laminectomy of C3 rather than a laminoplasty of C3 at the proximal end of the construct, it is possible to decompress the C2–3 and C3–4 motion segments without detaching the C2 muscle insertions at all. In contrast, when a C3 laminoplasty is performed, some detachment of the C2 insertions is often necessary to expose the distal lamina of C2 so that it can be removed and “un-shingled” off of the proximal lamina of C3 to allow for C3 opening. Using this approach, the average loss of lordosis was only 3° after C3 laminectomy, versus 9° when a C3 laminoplasty was performed at the proximal end of the construct (50).
Neck pain
Neck pain associated with laminoplasty is often cited as a reason for choosing an alternative procedure (18,25,29,51). However, recent studies have attempted to clarify the rates of persistent versus new-onset neck pain, and identify important independent predictors (52). Oshima et al. remind us that the development of neck pain is often influenced by a wide spectrum of variables and is likely multifactorial in nature (53). As discussed above, laminoplasty is not indicated for patients with significant preoperative neck pain complaints as they may have effective relief of symptoms (12,31,51).
In our view, however, laminoplasty does not lead to worsening axial neck pain in the properly selected patient. In our recent study, laminoplasty was performed in those who presented with neutral to lordotic C2–7 alignment and who did not complain of diffuse axial pain (20). Otherwise, laminectomy with fusion was performed. At average 18.5-month follow-up, neck pain scores did not worsen, but actually improved in both groups (not significant for laminoplasty; significant for laminectomy with fusion). Overall pain (including arm pain) improved significantly in both groups, as did mJOA scores. Neck Disability Index (NDI) improved significantly only in the laminoplasty group. On the basis of these findings, we concluded that, in a properly selected group of myelopathic patients without significant diffuse axial pain preoperatively and appropriate sagittal alignment, laminoplasty did not lead to worsening axial neck pain, and it was associated with significant improvements in other clinical and myelopathy outcomes (20). Additionally, Kato et al. showed that the preservation of soft tissue on the C2 and C7 can reduce rates of postoperative in neck pain in previously asymptomatic patients (54).
C5 palsy
Postoperative C5 palsy is most likely a multi-factorial phenomenon which can complicate an otherwise successful operation at relieving spinal cord compression (55). It is generally a motor-dominant dysfunction of the deltoid and or biceps muscles. This complication is not unique to laminoplasty, and may occur after any type of cervical decompressive operation, although most authors agree that its incidence is higher with posterior-based procedures. In one series of 630 patients undergoing a variety of multilevel cervical decompressive procedures, the overall rate of C5 palsy was 6.7%. When evaluated according to the operation performed, laminoplasty actually had the lowest rate (4.8%), followed by anterior corpectomy (5.1%) (56). Laminectomy and fusion had the highest rate of C5 palsy (9.5%), Other reports in the literature indicate that up to 13% of patients after laminoplasty will have some form of postoperative deltoid and/or bicep dysfunction (37,55,57-59).
The mechanism is debated in the literature, although most agree its etiology is multi-faceted. There is conflicting data on the benefit of prophylactic foraminotomy (57). When encountered, we generally take a conservative approach to treatment. Although there is no definitive evidence for the practice, we usually prescribe a steroid taper along with physical therapy. It is very important to counsel patients as to the occurrence of this complication preoperatively. Fortunately, most patients are able to regain purposeful motor function by 6–9 months post-operatively, although recovery can be variable and protracted in nature (37,55,57-59). Other nerve root palsies can occur, but do so much more infrequently.
Neurologic worsening
Spinal cord injury is a rare but devastating complication following any form of spinal cord surgery. Special consideration for the myelopathic patient undergoing laminoplasty include maintaining adequate cord profusion during opening of the hinge, and ensuring the hinge is adequately supported during application of internal fixation to avoid excessive recoil.
Despite adherence to meticulous technique and absence of intraoperative spinal cord trauma, there exists a rare subset of patients who may develop unexpected neurologic worsening after decompression. This phenomenon is poorly understood, but one mechanism may be related to increased free radicals in a hypervascular ischemia-reperfusion cycle after decompression (5,7,60). In a rat model, blockade of tetrodotoxin (TTX) sensitive sodium channels decreased this risk (61). Delayed worsening of myelopathy can also be seen in a subset of patients after either an initial period of improvement or stable function, despite absence of cord compression on imaging. Usually, we have found that this occurs in patients with pre-existing T2 cord signal change, but the pathophysiologic mechanism is unclear.
Recurrent stenosis
Recurrent stenosis, or premature closure was reported in early literature at rates up to 10%, and occurred most frequently at C5 or C6 levels (17,38). It can be definitively diagnosed on CT scan or MRI. It is associated more frequently with suture or bone graft techniques for fixation and is very unlikely after plating (36). Providers should consider this in the differential diagnosis of patients who experience post-operative worsening of myelopathy, especially in those who initially demonstrate improvement or stable function (62). Stenosis at levels adjacent to decompressed levels is also possible.
In conclusion, laminoplasty is best indicated in cervical myelopathy for patients with multilevel stenosis who have preserved sagittal alignment and minimal to no axial neck pain related to spondylosis (13). In that setting, expansion of the laminar arch can allow for direct and indirect decompression of the spinal canal. We generally avoid laminoplasty in patients with significant preoperative neck pain, kyphotic alignment and substantial instability. When compared to cervical laminectomy and fusion, advantages include avoiding fusion-related complications and the preservation of motion. Key technical pearls include meticulous extensor muscle management, with special attention being given to preserving the soft tissue attachments to C2 whenever possible. In the properly selected patient, outcomes are comparable, and in some studies superior, to other operations for CSM.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee A. Tan and Ilyas S. Aleem) for the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The series “Advanced Techniques in Complex Cervical Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. JM Rhee: royalties from Biomet and Stryker; is a member of a speakers’ bureau or has made paid presentations on behalf of Biomet/Zimmer, Medtronic, and Depuy; serves as a paid consultant to Biomet, Synthes; has received research or institutional support from Depuy, Johnson & Johnson Company, Kineflex, and Medtronic; and serves as a board member of the Cervical Spine Research Society. Receives royalties from Wolters-Kluwer. Royalties for a laminoplasty plate. DS Weinberg has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fehlings MG, Tetreault LA, Riew KD, et al. A Clinical Practice Guideline for the Management of Patients With Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Nonmyelopathic Patients With Evidence of Cord Compression. Global Spine J 2017;7:70S-83S. [Crossref] [PubMed]
- Veidlinger OF, Colwill JC, Smyth HS, et al. Cervical myelopathy and its relationship to cervical stenosis. Spine (Phila Pa 1976) 1981;6:550-2. [Crossref] [PubMed]
- Pavlov H, Torg JS, Robie B, et al. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology 1987;164:771-5. [Crossref] [PubMed]
- Nagata K, Yoshimura N, Hashizume H, et al. Physical performance decreases in the early stage of cervical myelopathy before the myelopathic signs appear: the Wakayama Spine Study. Eur Spine J 2019;28:1217-24. [Crossref] [PubMed]
- Tetreault LA, Karadimas S, Wilson JR, et al. The natural history of degenerative cervical myelopathy and the rate of hospitalization following spinal cord injury: an updated systematic review. Global Spine J 2017;7:28S-34S. [Crossref] [PubMed]
- Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain 1972;95:101-8. [Crossref] [PubMed]
- Joaquim AF, Sielatycki JA, Riew KD. Anterior surgical options for cervical spondylotic myelopathy. Indian Spine Journal 2019;2:33-41. [Crossref]
- Emery SE, Bohlman HH, Bolesta MJ, et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. J Bone Joint Surg Am 1998;80:941-51. [Crossref] [PubMed]
- Rhee JM, Basra S. Posterior surgery for cervical myelopathy: laminectomy, laminectomy with fusion, and laminoplasty. Asian Spine J 2008;2:114-26. [Crossref] [PubMed]
- Oyama M, Hattori S, Noriwaki N. A new method of posterior decompression. Chubuseisaisi 1973;16:792-4.
- Ju KL Zhou F, Rhee JM. Cervical Laminoplasty. In: Holly LT, Anderson PA. Essentials of Spinal Stabilization. Springer, 2017:113-25.
- Rhee J. Laminoplasty. In: Vaccaro A, Eck JC. Surgical Atlas of Spinal Operations. 1st edition. JP Medical Ltd., 2013:202-10.
- Saadat E, Heller JG, Rhee JM. Cervical laminoplasty. In: Rhee JM. editor. Emory’s Illustrated Tips and Tricks in Spine Surgery. 1st edition. Wolters Kluwer Health, 2019:35-45.
- Shaffrey CI, Wiggins GC, Piccirilli CB, et al. Modified open-door laminoplasty for treatment of neurological deficits in younger patients with congenital spinal stenosis: analysis of clinical and radiographic data. J Neurosurg 1999;90:170-7. [PubMed]
- Heller JG, Edwards CC 2nd, Murakami H, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine (Phila Pa 1976) 2001;26:1330-6. [Crossref] [PubMed]
- Yonenobu K, Hosono N, Iwasaki M, et al. Laminoplasty versus subtotal corpectomy. A comparative study of results in multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976) 1992;17:1281-4. [Crossref] [PubMed]
- Hirabayashi K, Watanabe K, Wakano K, et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976) 1983;8:693-9. [Crossref] [PubMed]
- Duetzmann S, Cole T, Ratliff JK. Cervical laminoplasty developments and trends, 2003-2013: a systematic review. J Neurosurg Spine 2015;23:24-34. [Crossref] [PubMed]
- Ratliff JK, Cooper PR. Cervical laminoplasty: a critical review. J Neurosurg 2003;98:230-8. [PubMed]
- Stephens BF, Rhee JM, Neustein TM, et al. Laminoplasty Does not Lead to Worsening Axial Neck Pain in the Properly Selected Patient With Cervical Myelopathy: A Comparison With Laminectomy and Fusion. Spine (Phila Pa 1976) 2017;42:1844-50. [Crossref] [PubMed]
- Kawakami M, Tamaki T, Ando M, et al. Preoperative instability does not influence the clinical outcome in patients with cervical spondylotic myelopathy treated with expansive laminoplasty. J Spinal Disord Tech 2002;15:277-83. [Crossref] [PubMed]
- Srivastava NK, Singh S, Chauhan SP, et al. Our technique of midsagittal splitting laminoplasty for compressive cervical myelopathy and its short-term results. Asian J Neurosurg 2016;11:206-13. [Crossref] [PubMed]
- Bartels RH, van Tulder MW, Moojen WA, et al. Laminoplasty and laminectomy for cervical sponydylotic myelopathy: a systematic review. Eur Spine J 2015;24 Suppl 2:160-7. [Crossref] [PubMed]
- Manzano GR, Casella G, Wang MY, et al. A Prospective, Randomized Trial Comparing Expansile Cervical Laminoplasty and Cervical Laminectomy and Fusion for Multilevel Cervical Myelopathy. Neurosurgery 2012;70:264-77. [Crossref] [PubMed]
- Klineberg E. Cervical spondylotic myelopathy: a review of the evidence. Orthop Clin North Am 2010;41:193-202. [Crossref] [PubMed]
- Matsunaga S, Sakou T, Nakanisi K. Analysis of the cervical spine alignment following laminoplasty and laminectomy. Spinal Cord 1999;37:20-4. [Crossref] [PubMed]
- Suk KS, Kim KT, Lee JH, et al. Sagittal alignment of the cervical spine after the laminoplasty. Spine (Phila Pa 1976) 2007;32:E656-60. [Crossref] [PubMed]
- Kimura I, Shingu H, Nasu Y. Long-term follow-up of cervical spondylotic myelopathy treated by canal-expansive laminoplasty. J Bone Joint Surg Br 1995;77:956-61. [Crossref] [PubMed]
- Cho SK, Kim JS, Overley SC, et al. Cervical Laminoplasty: Indications, Surgical Considerations, and Clinical Outcomes. J Am Acad Orthop Surg 2018;26:e142-52. [Crossref] [PubMed]
- Matsuoka Y, Suzuki H, Endo K, et al. Small sagittal vertical axis accompanied with lumbar hyperlordosis as a risk factor for developing postoperative cervical kyphosis after expansive open-door laminoplasty. J Neurosurg Spine 2018;29:176-81. [Crossref] [PubMed]
- Kato M, Namikawa T, Matsumura A, et al. Effect of cervical sagittal balance on laminoplasty in patients with cervical myelopathy. Global Spine J 2017;7:154-61. [Crossref] [PubMed]
- Sakai K, Yoshii T, Hirai T, et al. Cervical sagittal imbalance is a predictor of kyphotic deformity after laminoplasty in cervical spondylotic myelopathy patients without preoperative kyphotic alignment. Spine (Phila Pa 1976) 2016;41:299-305. [Crossref] [PubMed]
- Oya J, Burke JF, Vogel T, et al. The accuracy of multimodality intraoperative neuromonitoring to predict postoperative neurologic deficits following cervical laminoplasty. World Neurosurg 2017;106:17-25. [Crossref] [PubMed]
- Kim DH, Zaremski J, Kwon B, et al. Risk factors for false positive transcranial motor evoked potential monitoring alerts during surgical treatment of cervical myelopathy. Spine (Phila Pa 1976) 2007;32:3041-6. [Crossref] [PubMed]
- Riew KD, Raich AL, Dettori JR, et al. Neck Pain Following Cervical Laminoplasty: Does Preservation of the C2 Muscle Attachments and/or C7 Matter? Evid Based Spine Care J 2013;4:42-53. [Crossref] [PubMed]
- Rhee JM, Register B, Hamasaki T, et al. Plate-only open door laminoplasty maintains stable spinal canal expansion with high rates of hinge union and no plate failures. Spine (Phila Pa 1976) 2011;36:9-14. [Crossref] [PubMed]
- Kobayashi Y, Matsumaru S, Kuramoto T, et al. Plate Fixation of Expansive Open-Door Laminoplasty Decreases the Incidence of Postoperative C5 Palsy. Clin Spine Surg 2019;32:E177-82. [Crossref] [PubMed]
- Hirabayashi K, Satomi K. Operative procedure and results of expansive open-door laminoplasty. Spine (Phila Pa 1976) 1988;13:870-6. [Crossref] [PubMed]
- Yoon ST, Hashimoto RE, Raich A, et al. Outcomes after laminoplasty compared with laminectomy and fusion in patients with cervical myelopathy: a systematic review. Spine (Phila Pa 1976) 2013;38:S183-94. [Crossref] [PubMed]
- Matsumoto M, Watanabe K, Tsuji T, et al. Risk factors for closure of lamina after open-door laminoplasty. J Neurosurg Spine 2008;9:530-7. [Crossref] [PubMed]
- Yoshida M, Otani K, Shibasaki K, et al. Expansive laminoplasty with reattachment of spinous process and extensor musculature for cervical myelopathy. Spine (Phila Pa 1976) 1992;17:491-7. [Crossref] [PubMed]
- Kurokawa T, Tsuyama N, Tanaka H, et al. Enlargement of spinal canal by the sagittal splitting of the spinous process. Bessatsu Seikeigeka 1982;2:234-40.
- Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am 2013;95:549-54. [Crossref] [PubMed]
- Winslow C, Bode RK, Felton D, et al. Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest 2002;121:1548-54. [Crossref] [PubMed]
- Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976) 2013;38:2149-55. [Crossref] [PubMed]
- Devin CJ, Chotai S, McGirt MJ, et al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery: a multicenter analysis. Spine (Phila Pa 1976) 2018;43:65-71. [Crossref] [PubMed]
- Caroom C, Tullar JM, Benton EG Jr, et al. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976) 2013;38:1183-7. [Crossref] [PubMed]
- Gubin AV, Prudnikova OG, Subramanyam KN, et al. Role of closed drain after multi-level posterior spinal surgery in adults: a randomised open-label superiority trial. Eur Spine J 2019;28:146-54. [Crossref] [PubMed]
- Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976) 2013;38:S232-52. [Crossref] [PubMed]
- Michael KW, Neustein TM, Rhee JM. Where should a laminoplasty start? The effect of the proximal level on post-laminoplasty loss of lordosis. Spine J 2016;16:737-41. [Crossref] [PubMed]
- Kimura A, Shiraishi Y, Inoue H, et al. Predictors of persistent axial neck pain after cervical laminoplasty. Spine (Phila Pa 1976) 2018;43:10-5. [Crossref] [PubMed]
- Robins JM, Luo L, Mallallah F, et al. Skip laminectomy versus cervical laminectomy, an analysis of patient reported outcomes, spinal alignment and re-operation rates: The Leeds spinal unit experience (2008–2016). Interdiscip Neurosurg 2019;16:44-50. [Crossref]
- Oshima Y, Matsubayashi Y, Taniguchi Y, et al. Mental State Can Influence the Degree of Postoperative Axial Neck Pain Following Cervical Laminoplasty. Global Spine J 2019;9:292-7. [Crossref] [PubMed]
- Kato M, Nakamura H, Konishi S, et al. Effect of preserving paraspinal muscles on postoperative axial pain in the selective cervical laminoplasty. Spine (Phila Pa 1976) 2008;33:E455-9. [Crossref] [PubMed]
- Sasai K, Saito T, Akagi S, et al. Preventing C5 palsy after laminoplasty. Spine (Phila Pa 1976) 2003;28:1972-7. [Crossref] [PubMed]
- Nassr A, Eck JC, Ponnappan RK, et al. The incidence of C5 palsy after multilevel cervical decompression procedures: a review of 750 consecutive cases. Spine (Phila Pa 1976) 2012;37:174-8. [Crossref] [PubMed]
- Katsumi K, Yamazaki A, Watanabe K, et al. Can prophylactic bilateral C4/C5 foraminotomy prevent postoperative C5 palsy after open-door laminoplasty?: a prospective study. Spine (Phila Pa 1976) 2012;37:748-54. [Crossref] [PubMed]
- Imagama S, Matsuyama Y, Yukawa Y, et al. C5 palsy after cervical laminoplasty: a multicentre study. J Bone Joint Surg Br 2010;92:393-400. [Crossref] [PubMed]
- Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976) 2003;28:2447-51. [Crossref] [PubMed]
- Tetreault L, Tan G, Kopjar B, et al. Clinical and Surgical Predictors of Complications Following Surgery for the Treatment of Cervical Spondylotic Myelopathy: Results From the Multicenter, Prospective AOSpine International Study of 479 Patients. Neurosurgery 2016;79:33-44. [Crossref] [PubMed]
- Karadimas SK, Laliberte AM, Tetreault L, et al. Riluzole blocks perioperative ischemia-reperfusion injury and enhances postdecompression outcomes in cervical spondylotic myelopathy. Sci Transl Med 2015;7:316ra194. [Crossref] [PubMed]
- Wang HQ, Mak KC, Samartzis D, et al. "Spring-back" closure associated with open-door cervical laminoplasty. Spine J 2011;11:832-8. [Crossref] [PubMed]


