
Use of an endoscope for spinal intradural pathology
Introduction
The concept of direct spinal cord visualization by intradural endoscopy was introduced in medical practice in the 1930s (1,2). However, until recently, an endoscope had been too large or not reliable enough to be safely inserted into the intradural space (3), so spinal intradural endoscopy was not widely performed. Rather, the spinal endoscopic technique has evolved in the treatment of extradural pathology, including herniated discs and spinal canal stenosis, through minimally invasive methods (4). After recent technical advancements have led to create a small and flexible endoscope (3,5,6), the enthusiasm to apply such a novel device to intradural pathologies was revived. Before using an endoscope in clinical cases, cadaveric studies were conducted to verify the usefulness and safety of manipulation in the intradural space (3,7,8).
In this review, we summarized recent clinical reports describing how spinal intradural endoscopy has provided direct and magnified visions inside the spinal canal from small and limited exposure. More importantly, to discuss its usefulness, safety, and limitations, we categorized available literature as follows: cystic and inflammatory diseases in the subarachnoid space, tethered cord syndrome, intradural extramedullary tumors, spinal arteriovenous malformations (AVM), and percutaneous cordotomy for intractable pain (Table 1).
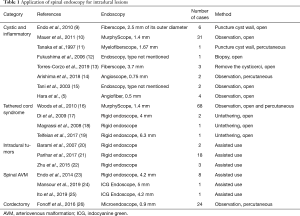
Full table
Cystic and inflammatory diseases in the subarachnoid space
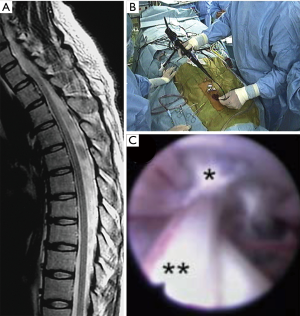
A flexible fiberscope that is sufficiently small can be passed through the dura into the intradural space with minimum incision. Based on an anatomical study, the sizes of the ventral and dorsal subarachnoid space around the spinal cord were 1–3 mm and 2–6 mm, respectively (3). Thus, an endoscope with an external diameter of <2 mm can safely pass along the spinal cord. Several authors indicated the usefulness of spinal endoscopy in cases of spinal arachnoid cysts (9-11). In surgery, following a few levels of hemilaminectomy, an endoscope was inserted into the cyst cavity through the dura and bone window (9). The endoscope was moved in the cranial and caudal directions to penetrate or remove the cyst wall. Intraoperative fluoroscopy helps surgeons confirm the position of the endoscope relative to the vertebral levels, which would provide important intraoperative feedback (9). Eventually, these maneuvers allowed to establish communications of the cyst cavity and the subarachnoid space, resulting in clinical improvements of neurological symptoms (Figure 1) (9). Although long-term follow-up is required to estimate recurrence rates, endoscopic treatment can be an important surgical intervention option.
The same strategy can be applied to subarachnoid inflammatory disease, in which observation and/or biopsy is required. Surgeons can observe a relatively long range along the dorsal and ventral spinal cord surface with an endoscope. In a case of suspected neurosarcoidosis, spinal endoscopic biopsy of the nodular lesion in the lumbar spinal cord established the diagnosis (12). Torres-Corzo et al. reported a rare case of neurocysticercosis, a parasitic disease affecting the human central nervous system, caused by the tapeworm Taenia solium (13). In this case, a flexible spinal endoscope was valuable to explore the entire subarachnoid space and remove parasites and cysticerci. Eventually, thickened adhesive arachnoid membranes were cleared, and cerebrospinal fluid (CSF) flow was restored under direct vision through an endoscope. Furthermore, cases of anterior located sacral meningocele and superficial siderosis were successfully treated with less invasive intradural spinal endoscopy (14,15).
These cases indicated that various intraspinal subarachnoid lesions, including arachnoid cysts and arachnoiditis, can benefit from spinal endoscopy. Especially when pathologies were extensive and extending over multiple vertebral levels in the subarachnoid space, open surgical treatment often require large incisions. A better alternative is to use a flexible endoscope that can be inserted and advanced through a small opening, allowing a less invasive approach.
Tethered cord syndrome
Endoscopy has also demonstrated diagnostic and therapeutic potential for tethered cord syndrome. In the lower lumbar vertebral level, an endoscope can be advanced through the cauda equina to observe the filum terminale, since the lumbar spine has a wider space compared to the thoracic or cervical vertebrae (3). Yörükoğlu et al. described a percutaneous fully endoscopic interlaminar approach to the filum terminale in cadaveric studies (7). In clinical cases, endoscopic observation was proven useful to visualize the filum terminale in 68 patients with tethered cord syndrome (16). Laminectomy and 2-mm dural incision was adequate to insert a flexible endoscope, which confirmed posterior displacement of the filum terminale, one of the diagnostic criteria of tethered cord syndrome (27).
Other authors described endoscopic untethering techniques for tethered cord syndrome (17-19). By interlaminar approach and 1-cm durotomy, a ridged endoscope could open the dura, coagulate and cut the filum terminale, and finally close the dura with continuous sutures. Although the number of the cases was small, a percutaneous endoscopic interlaminar approach may become a feasible option for untethering of the filum terminale in the near future.
Intradural extramedullary tumor
Barami et al. reported their first experience of assisted use of an endoscope to remove ventrally located intradural extramedullary tumors (20). Spinal endoscopy can effectively provide views of the ventral spinal cord without retraction, which was difficult through a microscope. Parihar et al. confirmed that endoscopic surgery can be applied to spinal tumors located in any spinal vertebral level (21). They could successfully remove 18 tumors, if maximal sagittal and axial diameters did not exceed 4.1 cm and 1.8 cm, respectively. Zhu et al. demonstrated the feasibility of endoscopic removal of intradural extramedullary tumors through an interlaminar approach (22). Vital structures, including an artery and affected spinal nerve roots, were dissected and safely coagulated using a bipolar flexible radiofrequency probe. When an endoscope is used in combination with an interlaminar approach, it would cause minimum bone destruction. Patients can benefit from less postoperative pain, minimal blood loss, and shorter recovery period. In such cases, individualized surgical planning and satisfactory dural closure technique is a key to success. A method to secure watertight CSF leak closure can enhance further application of spinal endoscopy in this type of surgery.
Spinal AVM
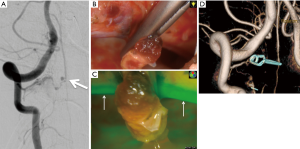
Endo et al. proposed other examples of utilizing angled endoscope in direct surgery for spinal AVM. For instance, perimedullary arteriovenous fistulas in the cervical spine are often located on the ventral surface of the spinal cord with close relationship to the anterior spinal artery (23). In such case, it would be difficult to visualize lesions under the microscope through a common posterolateral approach unless the spinal cord is extensively rotated. Instead, a combination of posterolateral exposure and assisted use of endoscopy can provide sufficient views of the ventral spinal cord without rotating it (24). Since the endoscopy was introduced following an open microsurgical procedure, hemilaminectomy and paramedian 2cm dural incision were required. By securing an adequate space to insert the rigid angled endoscope from posterolateral exposures, 360° circumferential views of the spinal cord surface can be appreciated with assisted use of an endoscope (23). When combined with indocyanine green (ICG) fluorescence endoscopy, it can further enhance its ability (24,25). Surgeons can appreciate detailed information regarding vascular anatomy and blood flow through ICG fluorescence endoscopy, which is important for proper management of spinal cord vascular lesions (Figure 2).
Intractable pains
Technical advancements have already resulted in smaller-diameter spinal endoscopes, making percutaneous use of an endoscope practical. Tanaka et al. used a percutaneous endoscope to perforate a spinal arachnoid cyst and restore CSF flow (11). The size of the endoscope is similar to that of a needle, therefore it can percutaneously puncture and provide a magnified view of the spinal cord surface. Fonoff et al. have contributed to the development of percutaneous endoscopic procedures for intractable pain (26,28). Using percutaneous dual channels, an endoscope could provide clear views of the pial surface of the spinal cord and tip of a radiofrequency cordotomy probe. Since surgeons appreciate real-time views, it became more reliable in determining targets for electrode insertion. Furthermore, trauma or injury of the spinal cord vessels or nerve roots is less likely to occur. As a result, procedures resulted in sufficient pain control with no complications of CSF leak (26). According to a recent review, cordotomy can be an optional method to treat cancer pain (29). As we consider that the increasing number of patients with cancer pain is related to minimal invasiveness and safety of endoscopic procedure, percutaneous endoscopic cordotomy can have wider clinical application.
Limitations
In this review, we focused on the usefulness of intradural spinal endoscopy, highlighting that both flexible and rigid angled endoscopes can provide views that are otherwise difficult to obtain through a microscope. However, limitations still exist regarding the degrees of manipulation that endoscopic instruments can offer. Thus, recent achievements regarding manipulation in endoscopic procedures were relatively confined to basic maneuvers, as pointed out elsewhere (30). The risk of bleeding and difficulty in obtaining hemostasis in endoscopic procedures should also be taken into account (22). Considering that technical difficulties may possibly be encountered during full endoscopic procedures, surgeons should be able to convert to a microsurgical procedure, if necessary. It can work as a backup and safeguard option in spinal intradural endoscopic surgery.
Another important issue is how to avoid postoperative CSF leak. In cases of endoscopic lumbar spinal surgery, the rate of dural tears was reported as high as 8.6% (31). Although there is no gold standard in managing dural tears or dural closure in endoscopic spine surgery, direct dural suturing techniques in endonasal surgery and in minimally invasive spine surgery could be helpful (32,33). If surgeons felt more comfortable in achieving water-tight dural closure under endoscopy, application of endoscopic spine procedures for both intradural and extradural pathologies would expand.
Conclusions
Existing literature confirmed increased utilization of spinal endoscopy in various intradural pathologies. An endoscope can provide direct views of the spinal cord from a small incision. By moving a fiberscope along the spinal cord, longitudinal lesions extending to multiple vertebral levels can be approached and surgically managed. Moreover, an endoscope can provide magnified views from different angles from a microscope. Using a rigid angled endoscope, even ventral spinal cord tumors or vascular lesions can be surgically managed by posterolateral approach. Technical advancements make percutaneous procedures possible in selected clinical indications. As experiences accumulate with further technical advancements, we believe that intradural endoscopy will be applied in the treatment of other diseases in this region.
Acknowledgments
The authors thank Enago (www.enago.jp) for the English language review.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2020.01.06). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burman M. Myeloscopy or the direct visualization of the spinal canal and its contents. J Bone Joint Surg 1931;13:695-6.
- Pool JL. Myeloscopy: intraspinal endoscopy. Surg Clin North Am 1957;37:1401-2. [Crossref] [PubMed]
- Shimada S, Tamaki N. Assessment of safety and feasibility of spinal endoscope in the thoracic and lumbar region: a cadaveric study. Kobe J Med Sci 2001;47:263-72. [PubMed]
- Kim M, Kim HS, Oh SW, et al. Evolution of Spinal Endoscopic Surgery. Neurospine 2019;16:6-14. [Crossref] [PubMed]
- Hara Y, Tamaki N, Nakamura M, et al. A new technique for intraoperative visual monitoring during spinal surgery: angiofiber and endoscopic ultrasonography. J Clin Neurosci 2001;8:347-50. [Crossref] [PubMed]
- Olinger CP, Ohlhaber RL. Eighteen-gauge needle endoscope with flexible viewing system. Surg Neurol 1975;4:537-8. [PubMed]
- Yörükoğlu AG, Tahta A, Akcakaya MO, et al. Percutaneous Fully Endoscopic Interlaminar Approach to the Filum Terminale: A Cadaveric Study. World Neurosurg 2016;92:402-6. [Crossref] [PubMed]
- Karakhan VB, Filimonov BA, Grigoryan YA, et al. Operative spinal endoscopy: stereotopography and surgical possibilities. Acta Neurochir Suppl 1994;61:108-14. [PubMed]
- Endo T, Takahashi T, Jokura H, et al. Surgical treatment of spinal intradural arachnoid cysts using endoscopy. J Neurosurg Spine 2010;12:641-6. [Crossref] [PubMed]
- Mauer UM, Gottschalk A, Kunz U, et al. Arachnoscopy: a special application of spinal intradural endoscopy. Neurosurg Focus 2011;30:E7. [Crossref] [PubMed]
- Tanaka T, Sakamoto T, Koyama T, et al. Endoscopic treatment of symptomatic spinal subarachnoid cysts. AJR Am J Roentgenol 1997;169:1719-20. [Crossref] [PubMed]
- Fukushima T, Shirota M, Yonemitsu T, et al. Neurological picture. Spinal endoscopic biopsy in the diagnosis of central nervous system neurosarcoidosis. J Neurol Neurosurg Psychiatry 2006;77:702. [Crossref] [PubMed]
- Torres-Corzo JG, Islas-Aguilar MA, Cervantes DS, et al. The Role of Flexible Neuroendoscopy in Spinal Neurocysticercosis: Technical Note and Report of 3 Cases. World Neurosurg 2019;130:77-83. [Crossref] [PubMed]
- Arishima H, Higashino Y, Yamada S, et al. Spinal endoscopy combined with selective CT myelography for dural closure of the spinal dural defect with superficial siderosis: technical note. J Neurosurg Spine 2018;28:96-102. [Crossref] [PubMed]
- Tani S, Okuda Y, Abe T. Surgical strategy for anterior sacral meningocele. Neurol Med Chir (Tokyo) 2003;43:204-9. [Crossref] [PubMed]
- Woods KR, Colohan AR, Yamada S, et al. Intrathecal endoscopy to enhance the diagnosis of tethered cord syndrome. J Neurosurg Spine 2010;13:477-83. [Crossref] [PubMed]
- Di X. Endoscopic spinal tethered cord release: operative technique. Childs Nerv Syst 2009;25:577-81. [Crossref] [PubMed]
- Magrassi L, Chiaranda I, Minelli M, et al. Total endoscopic approach to the cauda in a patient with a tight filum. Minim Invasive Neurosurg 2008;51:350-3. [Crossref] [PubMed]
- Telfeian AE, Punsoni M, Hofstetter CP. Minimally invasive endoscopic spinal cord untethering: case report. J Spine Surg 2017;3:278-82. [Crossref] [PubMed]
- Barami K, Dagnew E. Endoscope-assisted posterior approach for the resection of ventral intradural spinal cord tumors: report of two cases. Minim Invasive Neurosurg 2007;50:370-3. [Crossref] [PubMed]
- Parihar VS, Yadav N, Yadav YR, et al. Endoscopic Management of Spinal Intradural Extramedullary Tumors. J Neurol Surg A Cent Eur Neurosurg 2017;78:219-26. [Crossref] [PubMed]
- Zhu YJ, Ying GY, Chen AQ, et al. Minimally invasive removal of lumbar intradural extramedullary lesions using the interlaminar approach. Neurosurg Focus 2015;39:E10. [Crossref] [PubMed]
- Endo T, Shimizu H, Sato K, et al. Cervical perimedullary arteriovenous shunts: a study of 22 consecutive cases with a focus on angioarchitecture and surgical approaches. Neurosurgery 2014;75:238-49; discussion 249. [Crossref] [PubMed]
- Mansour A, Endo T, Inoue T, et al. Clipping of an anterior spinal artery aneurysm using an endoscopic fluorescence imaging system for craniocervical junction epidural arteriovenous fistula: technical note. J Neurosurg Spine 2019;26:1-6. [PubMed]
- Ito A, Endo T, Inoue T, et al. Use of Indocyanine Green Fluorescence Endoscopy to Treat Concurrent Perimedullary and Dural Arteriovenous Fistulas in the Cervical Spine. World Neurosurg 2017;101:814.e1-e6. [Crossref] [PubMed]
- Fonoff ET, Lopez WO, de Oliveira YS, et al. Microendoscopy-guided percutaneous cordotomy for intractable pain: case series of 24 patients. J Neurosurg 2016;124:389-96. [Crossref] [PubMed]
- Yamada S, Won DJ, Pezeshkpour G, et al. Pathophysiology of tethered cord syndrome and similar complex disorders. Neurosurg Focus 2007;23:E6. [Crossref] [PubMed]
- Fonoff ET, de Oliveira YS, Lopez WO, et al. Endoscopic-guided percutaneous radiofrequency cordotomy. J Neurosurg 2010;113:524-7. [Crossref] [PubMed]
- Raslan AM, Cetas JS, McCartney S, et al. Destructive procedures for control of cancer pain: the case for cordotomy. J Neurosurg 2011;114:155-70. [Crossref] [PubMed]
- Chern JJ, Gordon AS, Naftel RP, et al. Intradural spinal endoscopy in children. J Neurosurg Pediatr 2011;8:107-11. [Crossref] [PubMed]
- Müller SJ, Burkhardt BW, Oertel JM. Management of Dural Tears in Endoscopic Lumbar Spinal Surgery: A Review of the Literature. World Neurosurg 2018;119:494-9. [Crossref] [PubMed]
- Amano K, Okada Y, Kawamata T. Usefulness of the knot-tightener device following dural suturing in endonasal transsphenoidal surgery: technical report. Neurosurg Rev 2019;42:593-8. [Crossref] [PubMed]
- Tredway TL, Musleh W, Christie SD, et al. A novel minimally invasive technique for spinal cord untethering. Neurosurgery 2007;60:ONS70-4; discussion ONS4.


