
Unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis
Introduction
Degenerative lumbar canal stenosis (DLCS) is the most common indication for spinal surgery in the elderly population (1). Wide laminectomy with or without concomitant fusion procedures were considered as the standard surgical procedures for decades (2-4). This classical approach usually involves extensive soft tissue dissection, which would result in fatty degeneration, atrophy, and weakness of paraspinal muscles and lead to failed back surgery syndrome (5,6). Randomized controlled trials showed that fusion adds little value to decompression for DLCS (7,8). Considering the complications of spinal fusion and instrumentation, simple but adequate decompression is a more reasonable approach for patients who do not have the absolute indications for additional stabilization procedures (9).
For more than 20 years, minimally invasive (MI) spine surgeries have successfully treated patients with various lumbar spinal diseases (10-13). With the advancement of surgical instruments and endoscopic technology, MI spine surgeries have evolved rapidly from mini-open to tubular or percutaneous endoscopic approaches. Other than the potential benefits of MI approach (smaller wounds, diminished local pain, less blood loss, less postoperative wound pain, and shorter hospital stays), biomechanical studies have demonstrated the importance of the posterior column, including the interspinous ligaments, the facet joints, and the capsules, in maintaining spinal stability (14,15). Therefore, to minimize injury to the paraspinal muscles and the posterior stabilizing structures is the most critical concern for the long-term results (16).
Unilateral biportal endoscopic (UBE) decompression techniques is a percutaneous full endoscopic technique. It is performed through two separated small surgical wounds on either side of the spinous process. Unlike other endoscopic approaches, UBE is not confined by the working tube or the working channel. With continuous high-pressure normal saline irrigation and high-definition arthroscope, the surgeon can do very precise decompression in a clear and magnified surgical field.
This study is aimed to describe the UBE decompression techniques for DLCS with emphasis on how to safely perform adequate decompression while preserving the facet joints via the posterior interlaminar approach. The radiological and clinical outcomes were examined to evaluate the efficacy of this MI technique.
Methods
Patient selection
From July 2018 to Feb 2019, 81 consecutive patients with DLCS treated by UBE decompression techniques were retrospectively reviewed for this study. The indications of surgery were persistent radicular leg pain, neurological deficits, or neurogenic intermittent claudication refractory to conservative treatment for at least 6 months due to moderate to severe canal stenosis demonstrated by MRI. Exclusion criteria included: (I) pre-existing degenerative scoliosis with a Cobb’s angle more than 20 degrees, or more than grade I degenerative spondylolisthesis; (II) segmental instability, which was defined as translation of more than 4 mm or 10 degrees of angular motion between flexion and extension on upright lateral radiographs, or lateral bending on upright anteroposterior radiographs; (III) history of prior lumbar spine surgery.
They were 38 males and 43 females with an average age of 70.2±10.8 (range, 39–92). Of the 81 patients included in this study, 69 had canal stenosis and 12 patients had associated low grade spondylolisthesis. A total of 105 levels of decompression were done. Fifty-eight patients had 1-level decompression, 22 patients had 2-level decompression, and 1 patient had 3-level decompression. The decompression was performed at T11–T12 in 1 patient, L1–L2 in 1 patient, L2–L3 in 4 patients, L3–L4 in 28 patients, L4–L5 in 67 patients, and L5-S in 4 patients.
Surgical techniques
After induction of general anesthesia, the patient is placed prone with the abdomen free over the radiolucent Relton-Hall frame. The skin and the surgical field are prepared in the usual manners. UBE surgery is performed under continuous normal saline irrigation. It is critical to ensure that the final layer of draping is waterproof and a smooth drainage system for the saline outflow is properly set up. Without these precautions, the patient may be soaked by the cold normal saline and complicated with hypothermia.
In order to obtain a true anterior-posterior image, the fluoroscope should be tilted parallel to the disc space. The spinal levels of interest are determined using biplanar fluoroscope and marked on the skin. UBE decompression requires two small incisions through the deep fascia: a smaller one about 5–6 mm for insertion of arthroscope and continuous normal saline irrigation; a larger one about 8–10 mm for the outflow of normal saline, which is used as the instrument portal (Figure 1). An arthroscopic system with either a 0- or 30-degree scope is essential.
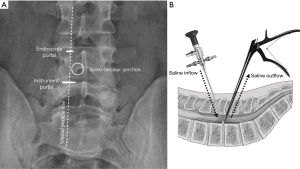
The initial target area for decompression is at the spino-laminar junction (the junction of spinous process and lower laminar margin of superior vertebra). The two skin incisions are usually located along the medial pedicle line, separated by 2–3 cm (Figure 1). We use serial dilators up to 10 mm to split the paraspinal muscles, enlarge the instrument portal, and gently detach the soft tissues off the interlaminar space. With the inflow of normal saline, a small space is created and ready to use. With meticulous hemostasis, the whole surgical procedure can be performed in a clear and magnified surgical field (Figure 2). Hemostasis for bleeding from small epidural veins and oozing from bones can be achieved by adjusting the inflow hydrostatic pressure and control of outflow. Bleeding from soft tissues and larger epidural veins can be cauterized efficiently by a radiofrequency wand (ArthroCare, Austin, Texas, USA). Bone wax is useful for stopping more severe bleeding from cancellous bone.
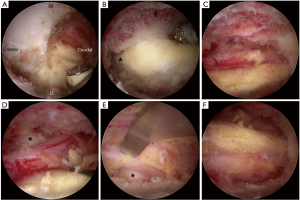
We always start the decompression from the spino-laminar junction using an electric high-speed diamond bur of 3 or 4 mm in diameter (Primado 2, NSK, Fukushima, Japan). The decompression procedures are performed according to the following steps (Figure 2):
- drill the ipsilateral lamina from its lower margin cranially until the origin of ligamentum flavum and underlying epidural fat are exposed (Figure 2A);
- separate the ligamentum flavum from the undersurface of contralateral lamina using a blunt neural dissector;
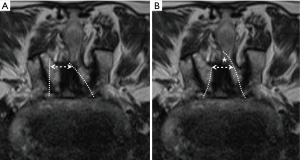
- drill the undersurface of contralateral lamina until the lateral recess is almost reached. Note that the ligamentum flavum must be preserved as a protector for underlying neural tissue. In the cases of severe stenosis, the spinous process and facet joints are usually hypertrophic and deformed. Removing more bone from the base of the spinous process would widen the laminotomy window and provide easier access to the contralateral lateral recess (Figures 2B,3);
- separate the contralateral ligamentum flavum from its attachment on the lamina, and decompress the contralateral lateral recess and foramen using small and curved Kerrison punches;
- remove the superficial layer of ligamentum flavum and preserve the deep layer as a protector;
- drill the upper laminar margin of the lower vertebra and then detach the ligamentum flavum from its caudal attachment;
- remove the contralateral half of the ligamentum flavum and decompress the lower surface of the contralateral facet joint to free the contralateral traversing nerve root (Figure 2C,D);
- perform ipsilateral decompression by drilling the medial margin of the ipsilateral lamina and facet joint. The facet drilling should be very conservative to preserve the facet joint as much as possible.
- remove the ipsilateral half of the ligamentum flavum, free the ipsilateral traversing nerve root, and check residual stenosis (Figure 2E);
- insert a small caliber suction drain tube after hemostasis.
Outcomes evaluation
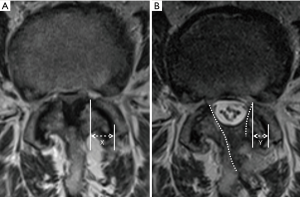
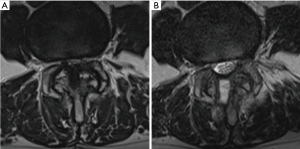
The pre-operative and final follow-up X-rays, including static and dynamic images, were reviewed to evaluate segmental instability. MRI studies of the lumbar spine was performed before surgery and 3 months after surgery. We measured the cross-sectional dural areas (CSDA) on the most stenotic axial MRI image to evaluate the decompression effect. Every measurement was repeated 3 times to get the mean value. Facet joint preservation was evaluated using the method described by Dohzono and Matsumura (17,18). However, MRI was used instead of CT scan (Figure 4).
Clinical outcome was evaluated using the visual analog scale (VAS) for back pain and lower leg symptoms, the Japanese Orthopedic Association (JOA) scores (18) for functional recovery, the Oswestry Disability Index (ODI) for degrees of disability, and modified MacNab criteria for overall treatment outcomes. These evaluations were performed before surgery and at the final follow-up. The medical charts were carefully reviewed to evaluate any complications, if existent.
Results
The average follow-up period was 8.6 months (range, 6–12 months). The operation time was 89±56.9 minutes (range, 50–190 minutes) per level of decompression. Intra-operative blood loss was minimal. The average hospital stay was 3.6±2.4 days (range, 3–6 days). Most of the patient got off the bed for ambulation on the 2nd post-operative day.
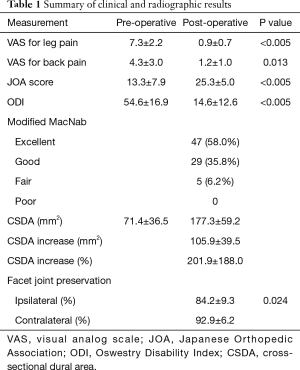
Significant improvement was obtained after the surgery. The VAS for leg pain was improved from 7.3±2.2 to 0.9±0.7 (P<0.005, paired t-test); VAS for back pain was also improved from 4.3±3.0 to 1.2±1.0 (P=0.013, paired t-test) at the final follow-up. The JOA score was improved from 13.3±7.9 to 25.3±5.0 (P<0.005, paired t-test) at the final follow-up. The average JOA improvement rate was 72.6%±40.0%. The ODI was improved from 54.6±16.9 to 14.6±12.6 (P<0.005, paired t-test). According to the modified MacNab criteria, the final outcomes were excellent in 47 patients (58.0%), good in 29 (35.8%), fair in 5 (6.2%), and poor in 0. That is, 92.6% of patients had good or excellent outcomes.
The stenotic spinal canal was significantly enlarged after UBE decompression. The measured CSDA at the most stenotic axial image on MRI was significantly increased from 71.4±36.5 to 177.3±59.2 mm2 (P<0.005, paired t-test). The average increment in CSDA was 105.9±39.5 mm2, corresponding to 201.9%±188.0% increase of pre-operative CSDA. The percentage of facet joint preservation was 84.2%±9.3% on the approach side and 92.9%±6.2% on the contralateral side. It was significantly higher on the contralateral side (P=0.024, paired t-test) (Figure 5). No patients had post-decompression segmental instability or progression of pre-existing spondylolisthesis. Facet joint effusion was noted in 3 patients (Table 1).
Full table
A few surgical complications were noted. Four patients had small dural tears. Direct repair under endoscope was performed successfully in 1 patient. The other 3 were treated conservatively. No cerebrospinal fluid leakage was encountered. All the 4 dura tears occurred in the first 30 patients. The other complications included transient motor weakness in 1 patient, epidural hematoma in 1 patient, and inadequate decompression in 1 patient. There was no infection or wound related complications.
Discussion
For the surgical treatment of DLCS, adequate decompression is the most critical determining factor. To avoid destruction to the posterior stabilizing structures, bilateral decompression via unilateral laminotomy was the most frequently used decompression method. In a biomechanical cadaver study, this decompression method was demonstrated to maintain more than 80% stiffness of the intact spine, and it can preserve the facet joints better than other decompression methods (19). In order to enhance recovery after surgery, various MI approaches (open, microscopic, tubular retractor assisted, microendoscopic, endoscopic assisted, or full-endoscopic) have been proposed to further minimize the surgical wounds and injury to the paraspinal muscles (10-13). However, the advantages of minimal invasiveness must be weighed against the drawbacks of limited visual field, limited working space, steep learning curve, radiation exposure, cost, compromised treatment results, and complications.
The concepts of the UBE decompression technique have been proposed since 2003 as an MI surgical technique for treatment of lumbar disc herniation and spinal canal stenosis. However, there was a paucity of development due to lack of handy instruments such as the radiofrequency wands for hemostasis and power motor drills for efficient removal of bony pathologies. With the advancement of endoscopic technology and surgical instruments in recent years, UBE techniques have been successfully applied on various disorders involving the cervical, thoracic, and lumbar spines (20-22). Because no tubular retractor is used to maintain the access portals, we can handle the instruments almost the same way as we do in the open surgeries (23). With meticulous hemostasis and proper control of hydrostatic pressure of normal saline, the surgical field is almost bloodless. The diameter of the endoscope is only 4 mm, which allows us to bring it very close to the pathology for a more precise decompression and delicate manipulation of the neural tissue.
Adequate decompression can be achieved with UBE decompression techniques. In our study, the average CSDA was increased from 71.4 to 177.3 mm2 with an average increase of 201.9%. Our clinical data also showed great improvements after the operation. The most significant one was VAS for leg pain, which was improved from 7.3 to only 0.9. The patients also had significant improvement in the neurological symptoms as well as the disability status, which were reflected by the improvement in JOA scores and ODI. In addition, more than 90% of patients had good or excellent outcomes as evaluated using the modified MacNab criteria.
The facet joints complex (including the synovial facet joint and the joint capsule) is the most important among the posterior stabilizing structures. Biomechanical tests have demonstrated that more than 50% of facet joint destruction can lead to segmental instability (24). All the MI approaches aim to obtain adequate decompression while preserving the integrity of the facet joint complex. When performing bilateral decompression through unilateral laminotomy, the approach side facet joint destruction was always a concern. Facet undercutting has been suggested to avoid excessive facet joint destruction. Using curved instruments including osteotomes, Kerrison punches, and high-speed drills might help reduce facet destruction (25). However, such techniques were difficult for open, tubular retractor assisted or microendoscopic approaches, because the surgeon’s visual point remained outside of the patient’s body or outside of the lamina. With an endoscopic approach, especially UBE, the surgeon’s visual point can be advanced inside of the lamina or into the contralateral lateral recess and the contralateral foramen. That feature enables precise check of the offending pathological structures without visual limitation. If a 30-degree endoscope is used, the visual field would be even wider.
In our study, the decompression was adequate, and the facet joints were preserved very well. Facet joint preservation was 92.9% on the contralateral side and 84.2% on the approach side. It is unavoidable that facet joint destruction is more severe on the approach side (17,18). For patients with severe stenosis, the spinous process and facet joints usually become hypertrophic and deformed. These deformities make the space between the spinous process and the facet joint very narrow. Bilateral decompression via unilateral laminotomy then becomes very difficult and may result in excessive destruction of the ipsilateral facet joint. Two modified approach techniques may solve these problems. First, do the contralateral side decompression first to create space for the neural tissue to mobilize contralaterally. Second, remove more bone at the base of the spinous process for easier sublaminar decompression and getting access to the contralateral recess. These modified techniques shift the laminotomy window contralaterally and minimize drilling on the ipsilateral lamina and facet joint (Figure 3).
The more facet joint is preserved, the less risk of instability after decompression. As compared with open laminectomy, the incidence of post-decompression segmental instability is significantly lower for MI decompression even in patients with pre-existing low-grade spondylolisthesis (26-28). In our study, there was no iatrogenic spondylolisthesis or progression of pre-existing spondylolisthesis in the very short follow up period. However, 3 patients developed facet joint effusion which was not noted in pre-operative MRI study. Because facet joint effusion is an indicator for segmental instability, longer follow-up is needed to reach a conclusion.
The learning curve for UBE decompression techniques is relatively shallow as compared with other MI decompression techniques, such as microendoscopic or percutaneous uniportal endoscopic techniques, estimated around 30 and 100 cases respectively (29-31). For a surgeon familiar with open surgeries but no experience of endoscopic procedures, the learning curve for UBE decompression is about 30 cases. However, for a surgeon familiar with microendoscopic or percutaneous endoscopic procedures, the learning curve can be reduced to 10 or 15 cases. The key points are to be familiar with control of the hydrostatic pressure and hemostasis skills in the small space with continuous normal saline irrigation. Dural tear is the most encountered complication in UBE decompression surgeries. Nevertheless, most of the time the dural tear is very small and conservative treatment is enough. Direct dural repair under the endoscope is possible but technically demanding (32). We did not have dural tear after the first 30 cases. Using blunt neural dissectors and high-speed diamond bur is much safer than using sharp curettes and osteotomes. Most of all, the ligamentum flavum is a perfect protector for the underlying neural tissue; it should not be removed until all the bony procedures are done.
Conclusions
The UBE decompression technique for DLCS is a safe and effective MI technique. Soft tissue destruction and the facet joint destruction can be minimized. It is therefore possible to avoid spinal fusion as well as to preserve the segmental mobility and stability. Moreover, the learning curve is less steep than for other MI decompression techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2020.03.08). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a retrospective study from chart review and image analysis. Therefore, the authors don't have formal ethical information for this study, and it is not mandatory in Far-Eastern Memorial Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008;358:794-810. [Crossref] [PubMed]
- Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am 1995;77:1036-41. [Crossref] [PubMed]
- Lee SY, Kim TH, Oh JK, et al. Lumbar Stenosis: A Recent Update by Review of Literature. Asian Spine J 2015;9:818-28. [Crossref] [PubMed]
- Rompe JD, Eysel P, Zollner J, et al. Degenerative lumbar spinal stenosis. Long-term results after undercutting decompression compared with decompressive laminectomy alone or with instrumented fusion. Neurosurg Rev 1999;22:102-6. [Crossref] [PubMed]
- Gille O, Jolivet E, Dousset V, et al. Erector spinae muscle changes on magnetic resonance imaging following lumbar surgery through a posterior approach. Spine (Phila Pa 1976) 2007;32:1236-41. [Crossref] [PubMed]
- Gejo R, Matsui H, Kawaguchi Y, et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine (Phila Pa 1976) 1999;24:1023-8. [Crossref] [PubMed]
- Peul WC, Moojen WA. Fusion for Lumbar Spinal Stenosis--Safeguard or Superfluous Surgical Implant? N Engl J Med 2016;374:1478-9. [Crossref] [PubMed]
- Forsth P, Olafsson G, Carlsson T, et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med 2016;374:1413-23. [Crossref] [PubMed]
- Yavin D, Casha S, Wiebe S, et al. Lumbar Fusion for Degenerative Disease: A Systematic Review and Meta-Analysis. Neurosurgery 2017;80:701-15. [Crossref] [PubMed]
- Kim HS, Paudel B, Jang JS, et al. Percutaneous Full Endoscopic Bilateral Lumbar Decompression of Spinal Stenosis Through Uniportal-Contralateral Approach: Techniques and Preliminary Results. World Neurosurg 2017;103:201-9. [Crossref] [PubMed]
- Mobbs RJ, Li J, Sivabalan P, et al. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine 2014;21:179-86. [Crossref] [PubMed]
- Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J 2009;18:672-8. [Crossref] [PubMed]
- Minamide A, Yoshida M, Yamada H, et al. Clinical outcomes after microendoscopic laminotomy for lumbar spinal stenosis: a 5-year follow-up study. Eur Spine J 2015;24:396-403. [Crossref] [PubMed]
- Lu WW, Luk KD, Ruan DK, et al. Stability of the whole lumbar spine after multilevel fenestration and discectomy. Spine (Phila Pa 1976) 1999;24:1277-82. [Crossref] [PubMed]
- Okawa A, Shinomiya K, Takakuda K, et al. A cadaveric study on the stability of lumbar segment after partial laminotomy and facetectomy with intact posterior ligaments. J Spinal Disord 1996;9:518-26. [Crossref] [PubMed]
- Bresnahan LE, Smith JS, Ogden AT, et al. Assessment of Paraspinal Muscle Cross-sectional Area After Lumbar Decompression: Minimally Invasive Versus Open Approaches. Clin Spine Surg 2017;30:E162-8. [Crossref] [PubMed]
- Dohzono S, Matsumura A, Terai H, et al. Radiographic evaluation of postoperative bone regrowth after microscopic bilateral decompression via a unilateral approach for degenerative lumbar spondylolisthesis. J Neurosurg Spine 2013;18:472-8. [Crossref] [PubMed]
- Matsumura A, Namikawa T, Terai H, et al. The influence of approach side on facet preservation in microscopic bilateral decompression via a unilateral approach for degenerative lumbar scoliosis. Clinical article. J Neurosurg Spine 2010;13:758-65. [Crossref] [PubMed]
- Hamasaki T, Tanaka N, Kim J, et al. Biomechanical assessment of minimally invasive decompression for lumbar spinal canal stenosis: a cadaver study. J Spinal Disord Tech 2009;22:486-91. [Crossref] [PubMed]
- Choi DJ, Kim JE, Jung JT, et al. Biportal Endoscopic Spine Surgery for Various Foraminal Lesions at the Lumbosacral Lesion. Asian Spine J 2018;12:569-73. [Crossref] [PubMed]
- Heo DH, Sharma S, Park CK. Endoscopic Treatment of Extraforaminal Entrapment of L5 Nerve Root (Far Out Syndrome) by Unilateral Biportal Endoscopic Approach: Technical Report and Preliminary Clinical Results. Neurospine 2019;16:130-7. [Crossref] [PubMed]
- Song KS, Lee CW, Moon JG. Biportal Endoscopic Spinal Surgery for Bilateral Lumbar Foraminal Decompression by Switching Surgeon's Position and Primary 2 Portals: A Report of 2 Cases With Technical Note. Neurospine 2019;16:138-47. [Crossref] [PubMed]
- Eun SS, Eum JH, Lee SH, et al. Biportal Endoscopic Lumbar Decompression for Lumbar Disk Herniation and Spinal Canal Stenosis: A Technical Note. J Neurol Surg A Cent Eur Neurosurg 2017;78:390-6. [Crossref] [PubMed]
- Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142-7. [Crossref] [PubMed]
- Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila Pa 1976) 2002;27:432-8. [Crossref] [PubMed]
- Guha D, Heary RF, Shamji MF. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg Focus 2015;39:E9. [Crossref] [PubMed]
- Asgarzadie F, Khoo LT. Minimally invasive operative management for lumbar spinal stenosis: overview of early and long-term outcomes. Orthop Clin North Am 2007;38:387-99. abstract vi-vii. [Crossref] [PubMed]
- Müslüman AM, Cansever T, Yılmaz A, et al. Midterm outcome after a microsurgical unilateral approach for bilateral decompression of lumbar degenerative spondylolisthesis. J Neurosurg Spine 2012;16:68-76. [Crossref] [PubMed]
- Choi DJ, Choi CM, Jung JT, et al. Learning Curve Associated with Complications in Biportal Endoscopic Spinal Surgery: Challenges and Strategies. Asian Spine J 2016;10:624-9. [Crossref] [PubMed]
- Nomura K, Yoshida M. Assessment of the Learning Curve for Microendoscopic Decompression Surgery for Lumbar Spinal Canal Stenosis through an Analysis of 480 Cases Involving a Single Surgeon. Global Spine J 2017;7:54-8. [Crossref] [PubMed]
- Lee CW, Yoon KJ, Kim SW. Percutaneous Endoscopic Decompression in Lumbar Canal and Lateral Recess Stenosis - The Surgical Learning Curve. Neurospine 2019;16:63-71. [Crossref] [PubMed]
- Pao JL. UBEST #4 dural repair [YouTube video] 2018.


