
Clinical presentation and diagnosis of delayed postoperative spinal implant infection
Introduction
One of the most significant complications of spinal surgery remains postoperative spinal implant infection (PSII), which leads to increased patient mortality and morbidity as well as poor long-term outcomes and high health-care costs (1). While in other orthopedic fields, such as arthroplasty, clear definitions, clinical diagnostic guidelines, and therapeutic algorithms have been established for periprosthetic joint infections (PJIs), these definitions, guidelines, and algorithms for spine surgery are still lacking even though with 0.7–20% the rate of postoperative infections is higher after spine surgery than after arthroplasty (2).
Depending on microbial virulence, PSII can either manifest early, typically within four weeks of surgery, or delayed, after more than four weeks up to years after surgery. These two types of infection differ in their clinical presentations, microbiological characteristics, and therapeutic strategies, which is why differentiation is highly important.
As the number of spine surgeries steadily increases, the number of PSII cases also rises. Currently, the diagnosis and treatment of PSII is mainly based on findings in arthroplasty. A clear definition as well as diagnostic and therapeutic algorithms are still missing for PSII. However, an understanding of its pathogenesis, risk factors, and treatment is essential to prevent or detect and successfully treat PSII. Here, we present published patient-related and surgical risk factors that need to be taken into account preoperatively, as well as clinical, laboratory, imaging and intraoperative examinations that need to be part of every perioperative workup for cases of suspected delayed infection to diagnose infection as early as possible and to initiate specific treatment.
PJI diagnostics
To best understand the diagnostics of delayed PSII, a brief overview of delayed infections in PJI is helpful. In arthroplasty, delayed infections are defined as typically occurring two months after implantation. They normally present with more subtle symptoms than acute infections, including joint pain or early loosening, and are caused by low-virulent organisms, such as coagulase-negative staphylococci or Cutibacterium species. Currently, a new working definition of PJI is under revision and includes clinical features (sinus tract or purulence around the prosthesis), leukocyte count in synovial fluid (>2,000/μL leukocytes or >70% granulocytes), periprosthetic tissue histology (inflammation with >23 granulocytes per 10 high-power fields) and microbiology (microbial growth in synovial fluid or in ≥2 positive tissue samples or in sonication fluid with >50 colony forming units/mL) (3). For the most accurate diagnosis of PJI, a combination of laboratory, histopathology, microbiology and radiology examinations is necessary (4). In arthroplasty, to differentiate between PJI and aseptic failure, preoperative joint aspiration needs to be performed for every painful prosthetic joint prior to revision surgery to determine the synovial fluid leukocyte count and percentage of granulocytes. Intraoperatively, three to five tissue samples should be submitted for culture and histopathology, and removed implants should be sent for sonication. Sonication fluid sample analysis was shown to have higher sensitivity than standard culture of periprosthetic tissue analysis (79% vs. 54%) (3).
Definition delayed infection
For delayed PSII, multiple definitions have been proposed in the literature. Most commonly, a delayed infection is defined as an infection occurring more than three months after the index surgery (5). Patients with delayed PSII usually present with chronic pain, implant failure or lack of adequate fusion several months to years after surgery (2). As symptoms and findings may not be distinct, diagnosis of delayed PSII can be difficult. Based on the abovementioned definition criteria for PJI, it has been suggested to define PSII as the presence of one or more of the following: (I) intraoperative purulence surrounding the tissue; (II) a sinus tract that communicates with the implant; (III) acute inflammation or peri-implant membrane type II or III according to Morawietz in the histopathological sample of peri-implant tissue; or (IV) a positive tissue or sonication culture, with the detection of low-virulent microorganisms in at least two samples, the detection of low-virulent microorganisms in at least one sample if the patient received antimicrobial treatment in the month prior to surgery, the detection of low-virulent microorganisms in one sample confirmed by the same microbial growth in the sonicate fluid culture or the detection of high-virulent microorganisms in at least one sample (Table 1) (6,7).
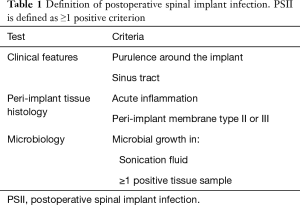
Full table
Microbiological characteristics
While in early postoperative spinal infections, virulent pathogens, such as Staphylococcus aureus, β-hemolytic streptococci, and aerobic gram-negative bacilli, are expected, in delayed PSII, low-virulent pathogens, such as coagulase-negative staphylococci (e.g., Staphylococcus epidermidis), Cutibacterium species and Propionibacterium acnes, are most frequently found (8). These bacteria are known to produce a polysaccharide biofilm and remain in a dormant state, which leads to resistance to host defenses and thereby mild or lack of systemic inflammation in the host (9).
Risk factors
Risk factors for postoperative spinal infections can be divided into patient-related and surgery-related risk factors. Patient-related risk factors include age, American Society of Anesthesiologists (ASA) score, diabetes mellitus, cardiovascular disease, obesity, smoking, malignancy, steroid use, previous lumbar surgery, nutritional status, chronic obstructive pulmonary disease, and immunologic competency. As surgery-related risk factors, duration of surgery, blood loss and blood transfusion, use of instrumentation, number of levels fused, surgical approach, and prolonged preoperative hospital stay have been identified to increase the risk for PSII (Table 2) (10). Instrumentation in particular leads to the adherence of microorganisms to its surface, which is aided by a polysaccharide biofilm. This biofilm not only reduces the efficiency of the host’s immune system but also antibiotic penetration and effectiveness. Additionally, instrumentation may cause metallosis, which in turn leads to granuloma formation (11).
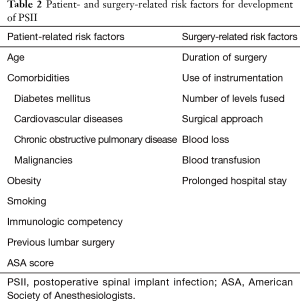
Full table
Clinical presentation
When considering a possible infection, the medical history should include the patient’s prior conditions and surgeries as well as the current course of symptoms. Symptoms of chronic infection are often difficult to distinguish from those of aseptic implant failure. 83% of all patients with delayed infection after spine surgery present with back pain, especially at the surgical site, and may report tenderness to palpation of the surrounding soft tissue (12). Fever may be present, but it is a less reliable symptom, as it occurs in only 16–65% of patients (13). Additionally, prolonged wound secretion of more than seven days may be a sign of delayed infection (14,15). However, in general, patients may have only vague complaints in cases of delayed infection, which is why in every revision surgery, infection must be considered as a possible diagnosis (16). The only definitive clinical signs of infection are visible purulence around the implant and the presence of a sinus tract (3).
Laboratory tests
In cases of suspected infection, initial blood work should include blood cultures, a complete blood count with a differential blood count, the erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Leukocyte elevation with a definite shift toward polymorphonuclear cells, an increasing ESR and elevated CRP are signs of postoperative infection (11). CRP normally peaks 2–3 days after surgery and returns to baseline within 2–3 weeks; ESR normally peaks around day 5 and returns to baseline over 3–6 weeks (17,18). However, only one single elevated value has low specificity for infection and can be high even without infection at any surgical site. In particular, an increasing trend rather than a single elevated value should be considered suggestive of postoperative infection (2). Additionally, a recent study showed a low sensitivity, at only 64%, and low specificity, at 68%, for serum CRP in detecting delayed PSII, especially when caused by low-virulent pathogens (19).
Imaging
Plain film radiography is usually the first imaging modality used in the detection of postoperative infection and can show lucency within the vertebrae, around grafts or around hardware as a sign of implant loosening and is especially helpful in inspecting surgical implants. While computed tomography (CT) can show implant position, bony changes and, to some extent, fluid collections (Figure 1), magnetic resonance imaging (MRI) is the most sensitive imaging modality, as it can show fluid collections, and should be used with gadolinium enhancement. Suspicious findings include rim-enhancing fluid collections, ascending epidural collections, bony destruction, and progressive marrow signal changes (16). However, since it is often not possible to distinguish purulent fluid from sterile seroma and thereby differentiate between postoperative changes and infection, the diagnosis of postoperative infection relies mostly on clinical presentation and laboratory tests (2,10).
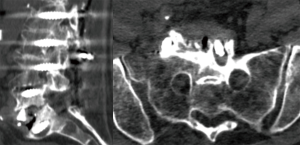
Intraoperative tissue culture and sonication
The gold standard for diagnosing PSII has been positive intraoperatively obtained tissue cultures. However, peri-implant tissue cultures are subject to potential contamination leading to false-positive results; additionally, recent studies have shown that similar to PJI, in the microbiological analysis of PSII sonication fluid, fluid culture is more sensitive than peri-implant tissue culture (6,20,21). The highest diagnostic accuracy was achieved by a combination of sonication fluid and peri-implant tissue cultures, reaching a sensitivity of 97% (6). Negative peri-implant tissue cultures may be explained by the abovementioned biofilm-forming properties of the causative microorganisms because they adhere to the implant’s surface, which is why the use of sonication improves the accuracy of PSII diagnosis (22). In the field of arthroplasty, it was shown that three tissue cultures should be obtained intraoperatively to achieve the highest sensitivity in detecting PJI (23). Additionally, peri-implant tissue should be sent for histopathological examination to determine peri-implant membranes, which are divided into the following types according to Morawietz: wear particle induced type (type I), infectious type (type II), combined type (type III), and indeterminate type (type IV) (7). Type I is characterized by foreign particles as well as an infiltration of macrophages and multinuclear giant cells, which form >20% of the membrane surface. Type II shows granulation tissue with neutrophilic granulocytes and plasma cells. Type III is a combination of the histomorphological changes in types I and II, and type IV is formed by connective tissue and lack the characteristics of types I and II (24).
Conclusions
To successfully treat delayed infections, PSII diagnosis and treatment need to be managed based on clinical guidelines and algorithms. Current practice often relies on findings from the field of arthroplasty, but these findings still need to be validated and optimized for spine surgery. The first step needs to be an internationally developed and accepted definition of PSII, similar to that of PJI.
However, spine surgeons need to be aware of known risk factors and diagnostic tools to detect these subtle delayed infections. It is important to note that in the majority of instrumented spine surgeries, several patient-related as well as surgery-related risk factors are present; therefore, one always needs to be cautious of the possible development of infection. Moreover, clinical, laboratory and radiological examinations have low sensitivity for the detection of delayed PSII. CRP is still used as the main laboratory screening parameter even though it may not show elevation in low-grade infections and has yet to be evaluated in a large meta-analysis of PSII. Similarly, imaging is usually not explicit since differentiation between infection and non-infection-related edema, hematoma or seroma is not always possible. In contrast to PJI, preoperative tissue sampling by joint aspiration is difficult in the spine due to its anatomy and has not yet been established. However, as Pumberger et al. showed in 2019, sonication culture in presumed aseptic spine revision surgeries was positive in 45.2% of cases (25). Therefore, for every painful or loose spine instrumentation, infection needs to be considered a possible diagnosis and ruled out by further diagnostic evaluations.
To achieve the highest sensitivity in the diagnosis of PSII, in every revision surgery, at least three intraoperative tissue samples should be submitted for culture, at least one tissue sample should be sent for histopathological examination, and the implant should be sonicated (6). Only by identifying the causative bacteria is optimal antibiotic treatment possible.
These guidelines do, however, still need validation by additional clinical studies. As in the field of arthroplasty, a clear definition of PSII and a diagnostic algorithm need to be established to detect as many infections as possible and to base a treatment algorithm on these findings.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Matthias Pumberger) for the series “Postoperative Spinal Implant Infection” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-499). The series “Postoperative Spinal Implant Infection” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sivasubramaniam V, Patel HC, Ozdemir BA, et al. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: a 15-year time-series study. BMJ Open 2015;5:e009011. [Crossref] [PubMed]
- Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 2013;4:S392-S403. [Crossref] [PubMed]
- Izakovicova P, Borens O, Trampuz A.. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev 2019;4:482-94. [Crossref] [PubMed]
- Trampuz A, Steckelberg JM, Osmon DR, et al. Advances in the laboratory diagnosis of prosthetic joint infection. Rev Med Microbiol 2003;14:1-14. [Crossref]
- Hedequist D, Haugen A, Hresko T, et al. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976) 2009;34:60-4. [Crossref] [PubMed]
- Bürger J, Akgün D, Strube P, et al. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur Spine J 2019;28:768-74. [Crossref] [PubMed]
- Krenn V, Morawietz L, Perino G, et al. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract 2014;210:779-86. [Crossref] [PubMed]
- Kowalski TJ, Berbari EF, Huddleston PM, et al. The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin Infect Dis 2007;44:913-20. [Crossref] [PubMed]
- Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 2012;65:158-68. [Crossref] [PubMed]
- Parchi PD, Evangelisti G, Andreani L, et al. Postoperative spine infections. Orthop Rev (Pavia) 2015;7:5900. [Crossref] [PubMed]
- Chaudhary SB, Vives MJ, Basra SK, et al. Postoperative spinal wound infections and postprocedural diskitis. J Spinal Cord Med 2007;30:441-51. [Crossref] [PubMed]
- Cunningham ME, Girardi F, Papadopoulos EC, et al. Spinal infections in patients with compromised immune systems. Clin Orthop Relat Res 2006.73-82. [Crossref] [PubMed]
- Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997;79:874-80. [Crossref] [PubMed]
- Viola RW, King HA, Adler SM, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine (Phila Pa 1976) 1997;22:2444-50. [Crossref] [PubMed]
- Bose B.. Delayed infection after instrumented spine surgery: Case reports and review of the literature. Spine J 2003;3:394-9. [Crossref] [PubMed]
- Meredith DS, Kepler CK, Huang RC, et al. Postoperative infections of the lumbar spine: presentation and management. Int Orthop 2012;36:439-44. [Crossref] [PubMed]
- Mok JM, Pekmezci M, Piper SL, et al. Use of C reactive protein after spinal surgery: Comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine (Phila Pa 1976) 2008;33:415-21. [Crossref] [PubMed]
- Thelander U, Larsson S.. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine (Phila Pa 1976) 1992;17:400-4. [Crossref] [PubMed]
- Akgün D, Bürger J, Pumberger M, et al. C-reactive protein misdiagnoses delayed postoperative spinal implant infections in patients with low-virulent microorganisms. Eur Spine J 2019;28:2990-5. [Crossref] [PubMed]
- Sampedro MF, Huddleston PM, Piper KE, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine 2010;35:1218-24. [Crossref] [PubMed]
- Saleh A, Guirguis A, Klika AK, et al. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty 2014;29:2181-6. [Crossref] [PubMed]
- Nana A, Nelson SB, McLaren A, et al. What’s new in musculoskeletal infection: update on biofilms. J Bone Joint Surg Am 2016;98:1226-34. [Crossref] [PubMed]
- Peel TN, Spelman T, Dylla BL, et al. Optimal periprosthetic tissue specimen number for diagnosis of prosthetic joint infection. J Clin Microbiol 2016;55:234-43. [Crossref] [PubMed]
- Morawietz L, Classen RA, Schröder JH, et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol 2006;59:591-7. [Crossref] [PubMed]
- Pumberger M, Bürger J, Strube P, et al. Unexpected positive cultures in presumed aseptic revision spine surgery using sonication. Bone Joint J 2019;101-B:621-4. [Crossref] [PubMed]


