
Comparative study between full-endoscopic laminectomy and microendoscopic laminectomy for the treatment of lumbar spinal canal stenosis
Introduction
Lumbar spinal canal stenosis (LSCS) is a common disease in the elderly, and considerable attention has been paid to its minimally invasive treatment (1). Microendoscopic laminectomy (MEL), which uses a 16-mm diameter tubular retractor and endoscope, is one of the established minimally invasive treatment methods of LSCS (2,3). Although there are small modifications to the approach (such as paramedian and midline approaches), MEL is a widely performed procedure, especially in Japan. Even in our hospital, almost all LSCS patients are treated using MEL. Recently, a 6.4-mm working channel endoscope for uniportal full-endoscopic spine surgery (FESS) became available in Japan. We therefore applied this system in the treatment of LSCS from June 2019.
Uniportal FESS was originally developed for the treatment of lumbar disc herniation and has recently been used for spinal canal stenosis (4-17). It was initially applied to treat foraminal and lateral recess stenosis; its application has now expanded to treating central type LSCS. Both technical refinements and the development of new instruments, such as a large working channel endoscope, have expanded FESS to the treatment of LSCS. However, the facilities where central-type LSCS with cauda equina symptoms can be treated using uniportal FESS are still limited. Furthermore, there are only a few studies comparing FESS and conventional operative strategies such as open, microscopic, or MEL (4,5,7,15). In this study, we retrospectively compared the operative outcomes of two different operative procedures (6.4-mm working channel FEL and MEL) and clarified the advantages and disadvantages of this new strategy.
We present the following article/case in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-620).
Methods
Study design: retrospective case-control study.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki and were in accordance with the ethical standards of the research committee of Iwai Medical Foundation. Informed consent was obtained from all patients via the disclaimer text on the internet home page of our hospital, according to the law of the Japanese Ministry of Health, Labour & Welfare.
Two hundred and seventeen consecutive patients with LSCS underwent posterior decompression using a 6.4-mm working channel endoscope (TOKOBO CO., LTD., Tokyo, Japan) or using the METRx endoscopic system (Medtronic Sofamor Danek, Memphis, TN, USA) between June 2019 and February 2020. All patients had cauda equina symptoms and/or radiculopathy resistant to medical treatment, epidural steroids, and/or nerve block. All patients had LSCS at only one vertebral level were treated via a paramedian approach using a 6.4-mm working channel endoscope. We excluded patients treated by MEL via a midline approach, because these two approaches have significant differences, even when using same endoscopic system (18). We also exclude patients treated by MEL at multi vertebral levels and patients treated by hemilaminectomy (without decompression of contralateral side). To concentrate on the surgical benefits for posterior decompression, we excluded patients who simultaneously underwent discectomy during both procedures. We also excluded patients in whom we could not distinguish that the radiculopathy was caused by the combined foraminal stenosis. We also excluded patients with lumbar spinal instability or moderate to severe spondylolisthesis (Meyerding classification: grade ≥ II). The instability was judged by gross motion (>3 mm) on flexion-extension lumbar lateral X-ray. In cases of severe degenerative scoliosis (coronal Cobb angle >15°), we also considered exclusion. Three patients treated using a 6.4-mm working channel endoscope dropped out because of the difficulty to accumulate follow-up data (Figure 1).
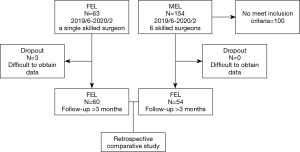
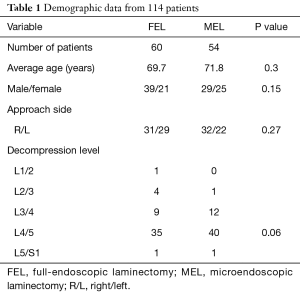
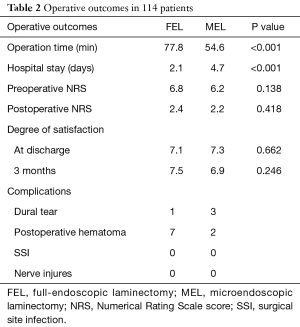
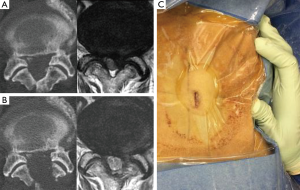
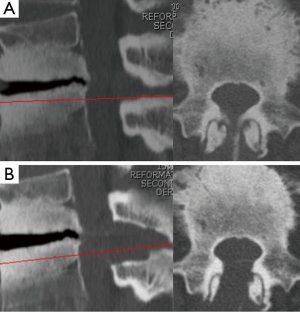
Background information of the patients, including age, sex, approach side, and the operated vertebral level, were obtained from medical records (Table 1). Operation time, postoperative hospital stay, and complications related to the operation, were also collected (Table 2). Neurological examination and preoperative computed tomography (CT) and T2-weighted magnetic resonance imaging (MRI) were used to identify the vertebral level of the LSCS and the target area for decompression. The extent of decompression was evaluated by performing pre- and postoperative CT and MRI (Figure 2A,B). Pre- and postoperative pain of the legs was evaluated using the Numerical Rating Scale (NRS) score. The postoperative NRS score was obtained at discharge from the hospital. The satisfaction score was also recorded at discharge and 3 months after the operation. The satisfaction score was then obtained by a medical clerk using an eleven-level rating scale, similar to the NRS (19). Statistical analysis was performed using Students’ t-test and Fisher’s exact test. P values less than 0.05 were considered statistically significant.
Full table
Full table
Surgical technique
The patients were carefully logrolled into the prone position. Surgery was performed under general anesthesia combined with motor evoked potential monitoring. During the operations, a fluoroscope was placed across the center of the operative table to ensure appropriate timing.
For MEL, surgery was conducted by six skilled surgeons. An 18-mm skin incision was made 10 mm lateral to the midline. The basic operative procedure was described previously (2,3). In addition to the basic paramedian approach, we mainly used a chisel (width: 4 mm) for bone removal (20,21).
For all FEL using a 6.4-mm working channel endoscope, surgery was conducted by a single skilled surgeon (H Koga). A 12-mm skin incision was made 10 mm lateral to the midline of the target vertebral level under fluoroscopic guidance (Figure 2C). The muscle attached to the lower margin of the cranial vertebral laminae (VL) and upper margin of the caudal VL was carefully detached using a dilator, in a similar manner to the operating technique for microendoscopic surgery (20,21). Next, an angled-working sheath and endoscope were inserted into the exposed VL and the VL was removed using a 4.0-mm diameter high-speed drill (NSK-Nakanishi Japan, Tokyo, Japan) across the cranial and caudal margin of the ligamentum flavum (LF). We could locate the central part of the LF that combined with the right and left LFs. The absence of adhesion to the underlying dura mater was confirmed using a dissector or blunt hook; the central part of the LF was then separated using a small curette and Kerrison rongeur. First, the ipsilateral LF was removed. To remove the LF as a single mass, it was also necessary to expose the lateral margins of the LF, as well as the cranial and caudal margins, using a high-speed drill and Kerrison rongeur. After removal of the inferior and superior articular processes (IAP and SAP), we could locate the lateral part of the epidural fat tissue or intact vertebral disc. After removal of all margins of the LF, we moved the LF and confirmed the absence of adhesion underneath the dura mater. If the dura mater moved together with the LF, we detached the dura mater from the LF using a dissector, blunt hook, and small curette. In such cases, we removed the non-adhesion area of the LF using a Kerrison rongeur, making it easier to detach the adhesion. Finally, we could visualize the underlying dura mater, nerve root, and vertebral disc (Figure 3).
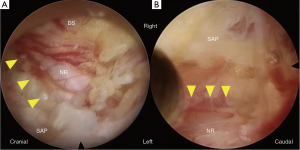
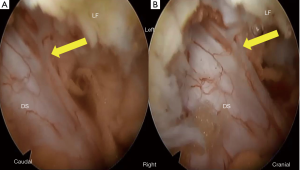
Second, the contralateral LF was removed. For the contralateral side, the dura mater and adjacent LF were always exposed in the operative field. We thus safely removed the inner layer of the contralateral VL before removal of the contralateral LF. After removal of the IAP and SAP, LF was easily removed using a Kerrison rongeur. If we found dural adhesion in this step, we carefully detached the dura mater from the inner LF surface (Figure 4) using dissector, blunt hook, and forceps. Finally, we could visualize the contralateral nerve root (Figure 3).
Bleeding from the epidural fat tissue and surface of the resected bone was electrocoagulated using a bipolar radio-frequency electrode system (Elliquence, Baldwin, NY, USA). After decompression, the endoscope and working sheath were carefully removed and the skin was closed using a single suture.
Results
Demographic data are summarized in Table 1. This case series consisted of 60 patients in the FEL group (male: 39, female: 21) and 54 in the MEL group (male: 29, female: 25). The mean age at surgery was 69.7 years in the FEL group and 71.8 years in the MEL group. The most commonly affected vertebral level was L4/5 in both groups (FEL: 58.3%, MEL: 74.1%). There were no significant differences in patient background between the FEL and MEL groups.
There was a significant difference in the mean operation time between the FEL group (77.8±18.8 min) and MEL group (54.6±17.6 min) (P<0.001). There was a significant difference in the mean postoperative hospital stay between the FEL group (2.13±1.38 days) and MEL group (4.74±1.67 days) (P<0.001) (Table 2). Regarding complications, seven patients in the FEL group and two in the MEL group were clinically diagnosed with postoperative hematoma, which presented with increasing low back and/or leg pain/paresthesia after surgery or removal of drainage. Although most patients recovered after conservative treatment, emergency hematoma evacuation was required for one patient in the FEL group. Dural tear was observed in one patient in the FEL group and three in the MEL group. Human fibrinogen compound (BOLHEAL) and a polyglycolic acid (NEOVEIL) sheet were used for repair in the MEL group, but direct repair was not performed in the FEL group. In both groups, patients exhibited no symptoms originating from the dural tear and were discharged within 8 days after the operation. No other postoperative complications, such as surgical site infection or nerve injures, were observed. The overall complication rate was 13.3% in the FEL group and 9.3% in the MEL group.
The preoperative NRS score in the FEL group of 6.8±1.8 improved significantly postoperatively to 2.5±1.9 (P<0.001) (Table 2). The preoperative NRS score in the MEL group of 6.2±2.4 also improved significantly postoperatively to 2.2±2.1 (P<0.001) (Table 2). There was no significant difference between the two groups. The mean satisfaction scores in FEL and MEL groups at discharge were 7.1±2.5 and 7.3±2.4, respectively. The mean scores in the FEL and MEL groups 3 months after the operation were 7.5±2.1 and 6.9±2.7, respectively. There was no significant difference between the two groups or different time points.
Discussion
Uniportal FESS was originally developed for the treatment of lumbar disc herniation and has recently been used for lumbar spinal canal stenosis (LSCS). Both technical refinements and the development of new instruments have expanded target diseases for FESS. However, studies using uniportal FESS for the treatment of LSCS have been limited (4-17). Furthermore, only a few comparative analyses with conventional operative procedures, such as open, microscopic, and MEL, have been reported (4,5,7,15). We therefore retrospectively compared the outcomes of FEL and MEL.
From our analysis, the effects on pain relief and postoperative satisfaction scores were almost identical between the FEL and MEL groups. Although FEL was superior in terms of a shorter postoperative hospital stay, the operation time was significantly longer than that of MEL (77.8 vs. 54.6 min). One of the reasons is that this case series is the initial 60 cases of FEL performed by one surgeon (H. Koga) and the procedure is technically difficult with a steep learning curve. As 77.8 min is not significantly different to previously reported operation times of MEL (77.0 and 66.1 min) using a high-speed drill (2,3), our MEL approach using a chisel (width: 4 mm) for bone removal might be faster than that of other groups. En bloc removal of the LF and hemostasis are sometimes the time-consuming steps of FEL. Further development of endoscopic instruments for these steps might reduce the operation time.
As for complications, it is possible to reduce dural tears in FEL. Because there is a clear operative field under saline irrigation in FEL, surgeons can confirm dural adhesion and the ligament between the LF and dura mater (referred to as the ATA or dorsal meningovertebral ligaments) (22,23). As the endoscope of FEL comes nearer to deeply located structures, such as the dura mater, than that of MEL, we can magnify those structures. Figure 4 clearly shows the presence of the ATA. Surgeons must carefully cut the ATA before LF removal, otherwise a dural tear may occur. The tilting and rotation techniques using oblique-viewing type endoscope have maximal merit when the endoscope comes as close as possible to the target structures (20,21).
The most critical complication of FEL is postoperative hematoma. We observed seven cases of symptomatic hematoma in FEL group. Only two cases were confirmed using postoperative MRI and myelo-CT, we clinically diagnosed the cases presenting with increasing low back and/or leg pain/paresthesia as hematoma. Although six patients recovered from the pain/paresthesia only with conservative treatment, we performed emergency evacuation in one case on the day of initial FEL. Compared with the other conventional procedures, the dead space in the dorsal area of the VL created by the FEL operative approach is extremely narrow. A small amount of hematoma may easily compress dural sac and/or nerve roots. In the case that require emergency evacuation, we only found a small blood clot. To prevent postoperative hematoma, intraoperative hemostasis seems to be most important. Especially bleeding from the surface of the resected bone should be persistently electrocoagulated using a bipolar radio-frequency electrode system (Video 1). If the bleeding from the bone cannot be stopped using the system, we recommend compressing the bone using a Kerrison rongeur (Video 2). It is also necessary to develop an instrument to put bone wax on the surface from a small working channel. Furthermore, postoperative hematoma in FEL frequently occurred with a delayed onset (several days after discharge, 4/7 cases); careful confirmation of hemostasis is important in the final stage of the operation. Reducing the pressure or stopping saline irrigation in the final stage may also help identify small bleeds.
The 6.4-mm endoscope has sufficient power to preserve the facet joint. The sharp angle of the facet joint on axial CT imaging is a frequently observed finding on LSCS, together with facet arthropathy. Especially for L1/2 and L2/3, partial facetectomy is sometimes performed during conventional decompressive laminectomy. The 6.4-mm working channel FEL can easily preserve a degenerated facet joint. It is a great advantage of this new strategy. Figure 5 shows the complete preservation of both L1/2 facet joints (Figure 4, right angle =79.7°, left angle =86.6°). Another advantage of the 6.4-mm working channel FEL is the ability to minimize the retraction of the dural sac and nerve root. As an endoscope was of the 30° oblique-viewing type and the lens was located on the top of the endoscope, we can visualize both nerve roots without retraction (L4/5 LSCS, Figure 3). This suggests that FEL is more minimally invasive than MEL, not only for the surrounding tissues (muscle, facet joint, and interspinous ligament), but also for nerve tissues.
We are also planning on applying FEL to multi-vertebral LSCS as reported by other investigators (6,13,16). However, the present 6.4-mm working channel FEL system is too short to perform two levels decompression through one small incision. On the other hand, MEL is possible to decompress two vertebral levels through one 18-mm incision. For a similar reason, we cannot perform FEL in moderately obese patients (BMI >30), but can perform MEL in moderately obesity patients. Therefore, we only perform FEL for single level LSCS in non-obese patients at the moment. The development of new instruments promises the application of FEL in these more complicated situations.
Conclusions
Preliminary results over a short follow-up period showed that the operative outcomes of 6.4-mm working channel FEL were not inferior to those of MEL for the treatment of LSCS. FEL is less invasive than MEL not only with respect to the surrounding tissues, such as the facet joint, but also the nervous tissue. Postoperative hematoma is the most critical complication of FEL and should be prevented using several hemostasis techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga, Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-620
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-620). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. HK served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Spine Surgery from Oct 2018 to Oct 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki and were in accordance with the ethical standards of the research committee of Iwai Medical Foundation. Informed consent was obtained from all patients via the disclaimer text on the internet home page of our hospital, according to the law of the Japanese Ministry of Health, Labour & Welfare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical Versus Nonsurgical Treatment for Lumbar Spinal Stenosis. Spine (Phila Pa 1976) 2016;41:E857-E868. [Crossref] [PubMed]
- Nomura K, Yoshida M. Assessment of the Learning Curve for Microendoscopic Decompression Surgery for Lumbar Spinal Canal Stenosis through an Analysis of 480 Cases Involving a Single Surgeon. Global Spine J 2017;7:54-8. [Crossref] [PubMed]
- Nomura K, Yoshida M. Microendoscopic Decompression Surgery for Lumbar Spinal Canal Stenosis via the Paramedian Approach: Preliminary Results. Global Spine J 2012;2:87-94. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine 2009;10:476-85. [Crossref] [PubMed]
- Heo DH, Lee DC, Park CK. Comparative Analysis of Three Types of Minimally Invasive Decompressive Surgery for Lumbar Central Stenosis: Biportal Endoscopy, Uniportal Endoscopy, and Microsurgery. Neurosurg Focus 2019;46:E9. [Crossref] [PubMed]
- Lee CW, Yoon KJ, Kim SW. Percutaneous Endoscopic Decompression in Lumbar Canal and Lateral Recess Stenosis - The Surgical Learning Curve. Neurospine 2019;16:63-71. [Crossref] [PubMed]
- Lee CW, Yoon KJ, Ha SS. Comparative Analysis Between Three Different Lumbar Decompression Techniques (Microscopic, Tubular, and Endoscopic) in Lumbar Canal and Lateral Recess Stenosis: Preliminary Report. Biomed Res Int 2019;2019:6078469.
- Lim KT, Nam HGW, Kim SB, et al. Therapeutic Feasibility of Full Endoscopic Decompression in One- To Three-Level Lumbar Canal Stenosis via a Single Skin Port Using a New Endoscopic System, Percutaneous Stenoscopic Lumbar Decompression. Asian Spine J 2019;13:272-82. [Crossref] [PubMed]
- Komp M, Hahn P, Merk H, et al. Bilateral Operation of Lumbar Degenerative Central Spinal Stenosis in Full-endoscopic Interlaminar Technique With Unilateral Approach. J Spinal Disord Tech 2011;24:281-7. [Crossref] [PubMed]
- Wang Y, Dou Q, Yang J, et al. Percutaneous Endoscopic Lumbar Decompression for Lumbar Lateral Spinal Canal Stenosis: Classification of Lateral Region of Lumbar Spinal Canal and Surgical Approaches. World Neurosurg 2018;119:e276-e283. [Crossref] [PubMed]
- Li YZ, Zhang HW, Zhang XG, et al. Efficacy and Safety of Percutaneous Endoscopic Decompression via Transforaminal and Interlaminar Approaches for Lumbar Spine Stenosis: Protocol for a Systematic Review and Meta-Analysis. Medicine (Baltimore) 2020;99:e18555. [Crossref] [PubMed]
- Wen B, Zhang X, Zhang L, et al. Percutaneous Endoscopic Transforaminal Lumbar Spinal Canal Decompression for Lumbar Spinal Stenosis. Medicine (Baltimore) 2016;95:e5186. [Crossref] [PubMed]
- Ito F, Ito Z, Shibayama M, et al. Step-by-Step Sublaminar Approach With a Newly-Designed Spinal Endoscope for Unilateral-Approach Bilateral Decompression in Spinal Stenosis. Neurospine 2019;16:41-51. [Crossref] [PubMed]
- Fujimoto T, Taniwaki T, Tahata S, et al. Patient outcomes for a minimally invasive approach to treat lumbar spinal canal stenosis: is microendoscopic or microscopic decompressive laminotomy the less invasive surgery? Clin Neurol Neurosurg 2015;131:21-5. [Crossref] [PubMed]
- Komp M, Hahn P, Oezdemir S, et al. Bilateral Spinal Decompression of Lumbar Central Stenosis With the Full-Endoscopic Interlaminar Versus Microsurgical Laminotomy Technique: A Prospective, Randomized, Controlled Study. Pain Physician. 2015;18:61-70. [PubMed]
- Kim HS, Paudel B, Jang JS, et al. Percutaneous full endoscopic bilateral lumbar decompression of spinal stenosis through uniportal-contralateral approach: techniques and preliminary results. World Neurosurg 2017;103:201-9. [Crossref] [PubMed]
- Hwang JH, Park WM, Park CW. Contralateral Interlaminar Keyhole Percutaneous Endoscopic Lumbar Surgery in Patients With Unilateral Radiculopathy. World Neurosurg 2017;101:33-41. [Crossref] [PubMed]
- Mikami Y, Nagae M, Ikeda T, et al. Tubular surgery with the assistance of endoscopic surgery via midline approach for lumbar spinal canal stenosis: a technical note. Eur Spine J 2013;22:2105-12. [Crossref] [PubMed]
- Tonosu J, Inanami H, Oka H, et al. Factors related to subjective satisfaction following microendoscopic foraminotomy for cervical radiculopathy. BMC Musculoskelet Disord. 2018;19:30. [Crossref] [PubMed]
- Baba S, Oshima Y, Iwahori T, et al. Microendoscopic Posterior Decompression for the Treatment of Thoracic Myelopathy Caused by Ossification of the Ligamentum Flavum: A Technical Report. Eur Spine J 2016;25:1912-9. [Crossref] [PubMed]
- Hayashi A, Oshima Y, Shiboi R, et al. Microendoscopic Posterior Decompression for the Treatment of Lumbar Lateral Recess Stenosis. J Spine 2016;5:4. [Crossref]
- Solaroglu I, Okutan O, Beskonakli E. The ATA and Its Surgical Importance: A Newly Described Ligament Lying Between the Dural Sac and the Ligamentum Flavum at the L5 Level. Spine (Phila Pa 1976) 2011;36:1268-72. [Crossref] [PubMed]
- Shi B, Li X, Li H, et al. The Morphology and Clinical Significance of the Dorsal Meningovertebra Ligaments in the Lumbosacral Epidural Space. Spine (Phila Pa 1976) 2012;37:E1093-E1098. [Crossref] [PubMed]


