
Complications associated with L4-5 anterior retroperitoneal trans-psoas interbody fusion: a single institution series
Introduction
Since the advent of lumbar interbody fusion in the 1940’s, multiple iterations and various techniques have been developed to gain access to the lumbar disc space. While early fusion techniques predominantly involved the posterior approach, contemporary minimally invasive surgical techniques have been introduced to reduce approach-related tissue trauma, decrease morbidity, enhance post-operative recovery, and improve long-term functional outcomes (1-8).
LLIF (lateral lumbar interbody fusion), or XLIF (Nuvasive)/DLIF (Medtronic) (extreme/direct lateral interbody fusion), technique involves direct access to the lateral disc space via a retroperitoneal trans-psoas approach and was first described in literature in 2006 by Ozgur et al. (9). This approach is most commonly indicated for degenerative conditions including spondylolisthesis, spinal deformity, and adjacent segment disease, but also has broad applications for trauma, tumor, infection, and revision surgery (3,10-14). In addition to its versatility, another primary advantage of the LLIF is the accommodation of a wide footprint interbody graft that spans the apophyseal rings of the intervertebral endplates, which augments stress shielding against subsidence, permits the delivery of a higher volume of bone graft, and improves fusion rates. Other advantages include the obviation of nerve root retraction, achievement of indirect neural element decompression, correction of sagittal and coronal mal-alignment, avoidance of paraspinous muscle disruption, reduced blood loss, decreased operative time, improved postoperative pain, and reduced length of stay (LOS) (15-18). With growing economic pressures driving lumbar fusion surgery to the ambulatory surgical setting, minimally invasive spine surgery (MISS), including LLIF, is becoming increasingly popular (19-21). Surgeons are leveraging the MISS applications of the trans-psoas approach, implementing this technique as a standalone lateral fusion construct. With the indirect decompression that is achieved, this can obviate the need for posterior decompression or instrumentation in appropriate cases (22-27).
Despite its advantages, the LLIF carries its own unique approach-related complications associated with collateral injury to the psoas muscle belly, which also harbors the lumbosacral plexus constituents. The most common complications of the LLIF include hip flexion weakness, anterior thigh dysesthesia/paraesthesias, femoral nerve injury, vascular injury, visceral injury, and pseudohernia (14). Despite intraoperative electromyographic monitoring, injury to the lumbosacral plexus, particularly the femoral nerve, is a feared and devastating complication of LLIF. In fact, due to the proximity and more ventral presence of the lumbosacral plexus to the surgical corridor, the lateral approach at L4-5 can be considered dangerous and remains controversial to some authors (18,28). Here we report our single-center, 3-year clinical experience of patients who underwent L4-5 LLIF. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-579).
Methods
The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board (IRB) of Houston Methodist Hospital (IRB # Pro00022592) and informed consent waiver was granted because of the retrospective nature of the study. A retrospective analysis of all patients who underwent LLIF at L4-5 between 2016 and 2019 was performed. Patients who underwent L5-S1 anterior lumbar interbody fusion (ALIF) in a supine position or transforaminal lumbar interbody fusion (TLIF) in a prone position were included, while patients who underwent LLIF at levels other than, or in addition to, L4-5 were excluded. Baseline demographics and clinical characteristics including age, sex, body mass index (BMI), indication for the procedure, medical comorbidities, tobacco status, operation performed, operative time, blood loss, LOS, discharge destination, intraoperative, and postoperative complications were recorded. To assess procedure-related complications, the peri-operative inpatient records and subsequent post-operative clinic notes were reviewed and changes in neurological exam and clinical symptoms were recorded. The motor exam was assessed by manual muscle testing and recorded using a 0–5 numerical scale where 0 and 5 indicate complete paralysis and full strength against resistance, respectively. A muscle strength of 4+ and 4− were converted to 4.5 and 3.5 accordingly to analyze changes in muscle strength. Postoperative imaging studies including X-ray or computed tomography (CT) of the lumbar spine were reviewed by a neuroradiologist to evaluate fusion.
Statistical analysis
Percentages were used to describe categorical variables while continuous variables were described by means and standard deviations. Univariate and multivariate logistic regression models were designed to analyze the relationship between the variables of interest and primary outcomes. All statistical analyses were performed using STATA 16 (StataCorp LLC, College Station, TX, USA). A minimum sample size of 100 was needed to achieve statistical significance and decrease type 1 error. Statistical analyses were performed on the basis of worst-case scenario with regard to loss to follow-up to decrease bias.
Results
During the 3-year study period, a total of 220 patients underwent L4-5 LLIF. The average age of the patients was 64.6±10.75. Fifty-eight percent of the patients were female, and the average BMI was 29.0±5.78 kg/m2. Twelve percent (n=26) of the patients had major cardiopulmonary medical comorbidities, such as coronary artery disease, previous myocardial infarction, or previous stroke, while 56.8% (n=125) of patients had minor medical comorbidities, such as hypertension, diabetes, or obstructive sleep apnea. Sixteen percent (n=36) of the patients were tobacco smokers. The percent of patients with previous spinal and non-spinal surgeries were 32.7% (n=72) and 84.1% (n=185), respectively. The most common indication for surgery was spondylolisthesis followed by degenerative disc disease. All but five patients underwent posterior transpedicular spinal instrumentation, with the majority of pedicle screws placed percutaneously (91.4%). Concurrent laminectomy, L5-S1 TLIF, and L5-S1 ALIF were performed in 69.5%, 23.6%, and 0.9% of the patients, respectively. The average cumulative operative time was 214 minutes and the average blood loss was 75 mL. The average LOS was 2.5 days and the majority (93.2%) of patients were discharged home. The baseline demographics and clinical characteristics are summarized in Tables 1 and 2, as well as Figure 1.
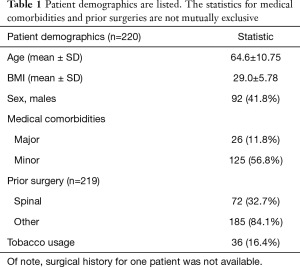
Full table
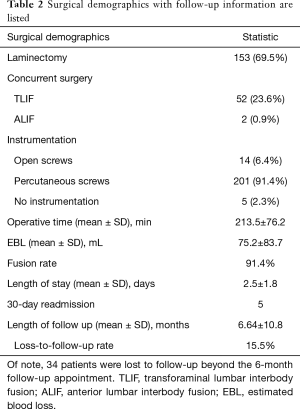
Full table
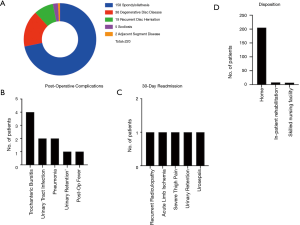
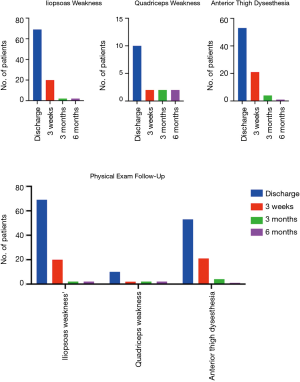
With regards to approach-related complications, 31.4% (n=69) and 9.1% (n=20) of patients experienced mild weakness (4 or 4+/5) in the ipsilateral iliopsoas at discharge and at 3-week postoperative visit, respectively. Mild quadricep weakness was observed in 4.5% (n=10) and 0.9% (n=2) of the patients at discharge and during the 3-week postoperative visit respectively. All patients with residual weakness at 3-week follow-up had weakness at discharge and there was no newly discovered weakness after discharge from the hospital (Figure 2). At 3 months post operation, only 2 patients (0.9%) had persistent residual mild (4+/5) iliopsoas and quadricep weakness on the side of the approach while all other patients were full strength. Post-operative anterior thigh dysesthesias and numbness were prevalent in 24.2% (n=53). During the 3-week and 3-month postoperative follow ups, the incidence of anterior thigh dysesthesias was reduced to 9.5% (n=21) and 1.8% (n=4), respectively. At 6-month follow-up, the incidence of iliopsoas/quadricep weakness remained unchanged (0.9%) while only 1 patient (0.5%) complained of numbness in the anterior thigh and groin on the side of the approach. The anterior thigh numbness in that patient was resolved at 9-month clinic follow-up. Other post-operative complications such as urinary tract infection, urinary retention, or trochanteric bursitis occurred in 5.9% (n=13) of patients as shown in Figure 1B. The 30-day all-cause hospital readmission rate was 2.3% (n=5) due to recurrent radicular pain, limb ischemia in a patient with a history of severe coronary artery and peripheral vascular disease, severe thigh pain on the approach side, urinary retention, and urosepsis (Figure 1C). Further multivariate logistic regression models revealed that female patients (OR =2.47), patients with higher BMI (OR =1.09), and patients with longer operative time (OR =1.01) were more likely to experience postoperative approach-related iliopsoas weakness at the time of discharge (P<0.05). Increased BMI was the only statistically significant factor associated with iliopsoas weakness 3 weeks postoperatively (OR =1.12, P<0.05). There were no statistically significant correlations between the variables and anterior thigh dysesthesias/pain. Complications are summarized in Figure 1B.
As seen in Table 2, the average length of follow-up was 6.6 months with 15.5% rate of loss-to-follow-up. The fusion rate inspected by either a computer tomography (CT) scan or a dynamic X-ray of the lumbar spine was 91.4%.
Discussion
In the recent decades, LLIF (lateral lumbar interbody and fusion) has gained increasing popularity due to the versatility of the technique and its potential to decrease surgical morbidity (11,29). However, the inherent risks associated with this approach, specifically at L4-5, have generated significant controversy regarding its safety (11,30,31). This study aims to report our single-center clinical experience with lateral interbody fusion at L4-5 and its associated outcomes and complications.
Hip flexion weakness and anterior thigh symptoms are common and approach-related postoperative findings ranging from 14% to 33% and are mostly due to psoas trauma caused by the retractor system (29,32-35). In our series, 31.4% and 9.1% of patients experienced transient hip flexion weakness at discharge and at 3-week postoperative follow up visit, respectively, while 99.1% of patients experienced normal hip flexor strength 3 months after surgery. Two patients (0.9%) with initial iliopsoas weakness at the time of discharge complained of persistent weakness (4/5) at 3- and 6-month follow up visits. The rate of anterior thigh symptom (numbness or pain) at discharge in our study was 24.2% (1.8% at 3 months, 0.5% at 6 months, and none at 9 months) compared to 27% reported by Hijji et al. (29). In our series only 2 patients experienced persistent approach-related iliopsoas and quadricep weakness at 3 and 6 months while the remainder of post-operative iliopsoas and quadricep weaknesses were transient in nature and all resolved prior to the 3-month follow up visit. The transient nature of these deficits indicates approach-related trauma to the psoas muscle and the embedded nervous structures as the likely etiology. The remaining 2 patients with persistent mild iliopsoas and quadricep weakness (4/5 iliopsoas and 4+/5 quadricep) at 6-month follow-up both had normal intraoperative neuromonitoring at the time of surgery. This is in concordance with results published by Houten et al. reporting 2 cases of persistent postoperative iliopsoas and quadricep weakness after L4-5 LLIF despite normal intraoperative neuromonitoring signals (33). Both of our patients were unfortunately lost to follow-up beyond the 6-month postoperative visit and no further data were available. Slight heterogeneity in data is anticipated due to inclusion of concurrent procedures such as TLIF (n=52) and ALIF (n=2) at L5-S1 as one may relate postoperative lower extremity paresthesias to radiculitis from the TLIF approach. Our subgroup analysis did not show any statically significant differences in postoperative leg dysesthesias in patients undergoing concurrent TLIF/ALIF. Moreover, the anatomic pattern of pain distribution caused by postoperative radiculitis at L5-S1 typically extends below the knee, which is different from the anterior thigh symptoms experienced after a transpsoas approach.
In a large systematic literature review, Hijji et al. reported that transient neurologic complications including thigh symptoms and motor weakness are the most common risks of the lateral approach (up to 36%) (29). Our clinical analysis at L4-5 shows similar results. However, one of the most feared complications of the lateral approach is persistent neurologic complications including lumbosacral plexopathy, femoral neuropathy, and motor or sensory loss in the anterior thigh. Based on the most comprehensive systematic review available to date, the overall risk of permanent neurologic deficit after a LLIF is 4%, which is higher than what was found in our study (0.9%) (29). In a separate retrospective study, Cahill et al. reported two incidents of femoral nerve injury in 201 patients, both during the dilation of the retractor at L4-5. The procedure was aborted at that level in both patients due to the anterior positioning of lumbosacral plexus. Both patients experienced profound weakness in the ipsilateral iliopsoas and quadricep muscle groups postoperatively. While one patient had full recovery at 3 months, the other reported persistent residual motor deficit (36).
Interestingly, other studies have reported statistically significant increased rate of persistent neurologic deficit with the use of rhBMP-2, fusion at L2-3 level, and higher number of levels treated (14,30,37). Our study investigated the complication rate after a one-level fusion at L4-5 and rhBMP was only used in 4.1% (n=9) of cases with no correlation to postoperative complications. Our study showed higher BMI, female sex, and length of operation as the factors associated with an increased risk of transient neurologic deficit after L4-5 LLIF. In our interpretation of this data, higher BMI and longer operative time may in part reflect longer retractor time and higher risk of injury to the nervous structures in the operative field. In our practice, “retractor time” or “psoas time” is defined as the time spent between intradiscal shim placement and retractor system removal. While retractor time was not collected in this study, our unpublished prospective clinical experience with 30 consecutive L4-5 LLIF revealed an average retractor time of 00:17:42, with times ranging from 12 to 32 minutes depending on disc height and anatomic complexity.
Aside from the known neurologic complications, patients undergoing the anterior retroperitoneal transpsoas approach are prone to vascular and visceral injuries that are well-reported in the literature (17,29). In our study, no patients suffered any vascular, bowel, or urologic injury.
In order to minimize operative complications associated with LLIF, specific preoperative evaluations and intraoperative measures are necessary to assure optimized outcomes and minimized morbidity. Below are some of the important steps involved in surgical planning and execution.
Radiographic analysis
In order to assure feasibility of the lateral approach at L4-5, it is critical to review the AP view of the lumbar X-ray and evaluate the iliac crest. At times, anatomy of the crest dictates the laterality of approach. If the anatomy of the iliac crest is marginally favorable, angled instruments can be considered to facilitate the operation. The anatomy of the psoas muscle on axial MRI is another important radiographic marker to consider when planning a transpsoas approach. A rising psoas sign or “Mickey Mouse” psoas refers to more ventral position of the psoas muscle with respect to the vertebral body and has been associated with higher risk of transient and permanent neurologic injury (18). The psoas muscle spans from T12 to L5 with gradual ventral migration rostro-caudally and complete dissociation from the vertebral body at L5-S1 disc level. According to a study by Kepler et al., more anterior position of the psoas muscle correlated with more anterior position of the lumbar plexus and in turn, higher incidence of iatrogenic neurologic injury (28). In cases of L4-5 LLIF, the surgeon should also pay close attention to lumbarization of sacral spine as the transitional levels seldom offer a safe surgical corridor to dock the retractor and will most likely necessitate an alternative approach.
Exposure of disc space and retractor docking
Direct inspection of the surgical corridor and avoidance of sharp dissection minimize the risk of neurologic and visceral injuries inherent to the approach. Moreover, safe surgical corridors have been described in several cadaveric and clinical studies. While exposure of L2-3 does not pose any risks of nerve injury, the genitofemoral nerve is anterior and superficial to the psoas muscle at L3-4 and L4-5 (38). Additionally, multiple studies have shown the dorsoventral migration of the lumbosacral plexus traveling from L2 to L5. One should especially be cognizant of the ventral migration at L4-5 as the plexus can be located as far anterior as at the midpoint of the disc space (36,39,40). In a cadaveric study, Uribe et al. divided the disc space equally into 4 segments, defined as zones I through IV going from anterior to posterior. From L2 to L5 the femoral nerve traverses the psoas muscle in a gradual dorsoventral trajectory and is frequently found in the posterior middle quarter (zone III) of the L4-5 disc space. Results of this study indicated that zone III is generally a safe surgical zone at all levels in patients without significant spondylolisthesis or coronal deformity (41). Finally, attention should be given to effective anchoring of the retractor to the vertebral body during deployment, particularly in the presence of osteophytes, to prevent unintentional migration of the retractor and possible risk of nerve injury (15,33,42).
Retractor time and surgeon experience
The process of retractor deployment and dilation is traumatic to the psoas muscle as well as the nervous structures buried within and every attempt should be made to minimize retractor time (34). Multiple studies describe the learning curve associated with minimally invasive spinal surgery and its effect on operative time. Literature shows that operative time reduces as a surgeon becomes more experienced with the approach, generally after performing the first 30 cases (43-45). We speculate that minimally invasive LLIF is not an exception to this rule. Moreover, excessive dilation of the retractor has been identified as a risk factor for approach-related nerve injury and should be avoided (34).
The current study reports our single center experience with L4-5 LLIF and its associated complications. There are several limitations to our study including its retrospective nature, relatively high rate of loss to follow-up, and incomplete operative data such as retractor time and number of aborted procedures. With regards to the high rate of loss to follow-up, the majority of this subgroup is comprised of out-of-town patients (n=24) that traveled to our institution for the operation. These patients were generally managed on an as-needed basis beyond the 3-month follow-up appointment (except for the 12-month imaging to evaluate arthrodesis) and therefore partially contributed to the relatively high rate of loss to follow-up. Finally, our study did not account for socioeconomic factors and their possible confounding effects on postoperative LOS, and therefore results are subject to bias. Nonetheless, our case series demonstrates favorable feasibility and safety of LLIF at L4-5 and warrants further evaluation of this approach via larger prospective multi-center studies.
Conclusions
Overall, our results suggest that the minimally invasive lateral approach proves to be a safe and effective technique at L4-5 to address a wide array of spinal pathologies. The complications inherent to the lateral transpsoas approach are comparable to other retroperitoneal interbody fusion approaches such as ALIF and OLIF. Surgeons should be mindful of factors contributing to increased risk of transient or permanent approach-related nerve injury including transitional anatomy, radiographic warning signs, retractor time, and degree of retractor dilation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-579
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-579
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-579). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board (IRB) of Houston Methodist Hospital (IRB # Pro00022592) and informed consent waiver was granted because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee LY, Idris Z, Beng TB, et al. Outcomes of Minimally Invasive Surgery Compared to Open Posterior Lumbar Instrumentation and Fusion. Asian J Neurosurg 2017;12:620-37. [Crossref] [PubMed]
- Park Y, Seok SO, Lee SB, et al. Minimally Invasive Lumbar Spinal Fusion Is More Effective Than Open Fusion: A Meta-Analysis. Yonsei Med J 2018;59:524-38. [Crossref] [PubMed]
- Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg 2014;82:230-8. [Crossref] [PubMed]
- Patel AA, Zfass-Mendez M, Lebwohl NH, et al. Minimally Invasive Versus Open Lumbar Fusion: A Comparison of Blood Loss, Surgical Complications, and Hospital Course. Iowa Orthop J 2015;35:130-4. [PubMed]
- Skovrlj B, Belton P, Zarzour H, et al. Perioperative outcomes in minimally invasive lumbar spine surgery: A systematic review. World J Orthop 2015;6:996-1005. [Crossref] [PubMed]
- Udeh BL, Costandi S, Dalton JE, et al. The 2-year cost-effectiveness of 3 options to treat lumbar spinal stenosis patients. Pain Pract 2015;15:107-16. [Crossref] [PubMed]
- Vertuani S, Nilsson J, Borgman B, et al. A Cost-Effectiveness Analysis of Minimally Invasive versus Open Surgery Techniques for Lumbar Spinal Fusion in Italy and the United Kingdom. Value Health 2015;18:810-6. [Crossref] [PubMed]
- Wang X, Borgman B, Vertuani S, et al. A systematic literature review of time to return to work and narcotic use after lumbar spinal fusion using minimal invasive and open surgery techniques. BMC Health Serv Res 2017;17:446. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Castro C, Oliveira L, Amaral R, et al. Is the lateral transpsoas approach feasible for the treatment of adult degenerative scoliosis? Clin Orthop Relat Res 2014;472:1776-83. [Crossref] [PubMed]
- Kwon B, Kim DH. Lateral Lumbar Interbody Fusion: Indications, Outcomes, and Complications. J Am Acad Orthop Surg 2016;24:96-105. [Crossref] [PubMed]
- Lehmen JA, Gerber EJ. MIS lateral spine surgery: a systematic literature review of complications, outcomes, and economics. Eur Spine J 2015;24 Suppl 3:287-313. [Crossref] [PubMed]
- Mummaneni PV, Shaffrey CI, Lenke LG, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus 2014;36:E6. [Crossref] [PubMed]
- Salzmann SN, Shue J, Hughes AP. Lateral Lumbar Interbody Fusion-Outcomes and Complications. Curr Rev Musculoskelet Med 2017;10:539-46. [Crossref] [PubMed]
- Benglis DM, Elhammady MS, Levi AD, et al. Minimally invasive anterolateral approaches for the treatment of back pain and adult degenerative deformity. Neurosurgery 2008;63:191-6. [Crossref] [PubMed]
- Dakwar E, Cardona RF, Smith DA, et al. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus 2010;28:E8. [Crossref] [PubMed]
- Isaacs RE, Hyde J, Goodrich JA, et al. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35:S322-30. [Crossref] [PubMed]
- Voyadzis JM, Felbaum D, Rhee J. The rising psoas sign: an analysis of preoperative imaging characteristics of aborted minimally invasive lateral interbody fusions at L4-5. J Neurosurg Spine 2014;20:531-7. [Crossref] [PubMed]
- Basques BA, Ferguson J, Kunze KN, et al. Lumbar spinal fusion in the outpatient setting: an update on management, surgical approaches and planning. J Spine Surg 2019;5:S174-S180. [Crossref] [PubMed]
- Chin KR, Pencle FJ, Coombs AV, et al. Lateral Lumbar Interbody Fusion in Ambulatory Surgery Centers: Patient Selection and Outcome Measures Compared With an Inhospital Cohort. Spine (Phila Pa 1976) 2016;41:686-92. [Crossref] [PubMed]
- Smith WD, Wohns RN, Christian G, et al. Outpatient Minimally Invasive Lumbar Interbody: Fusion Predictive Factors and Clinical Results. Spine (Phila Pa 1976) 2016;41 Suppl 8:S106-22. [PubMed]
- Aichmair A, Alimi M, Hughes AP, et al. Single-Level Lateral Lumbar Interbody Fusion for the Treatment of Adjacent Segment Disease: A Retrospective Two-Center Study. Spine (Phila Pa 1976) 2017;42:E515-E522. [Crossref] [PubMed]
- Hsieh PC, Koski TR, O'Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 2007;7:379-86. [Crossref] [PubMed]
- Inoue S, Watanabe T, Hirose A, et al. Anterior discectomy and interbody fusion for lumbar disc herniation. A review of 350 cases. Clin Orthop Relat Res 1984.22-31. [PubMed]
- Oxland TR, Lund T. Biomechanics of stand-alone cages and cages in combination with posterior fixation: a literature review. Eur Spine J 2000;9 Suppl 1:S95-101. [Crossref] [PubMed]
- Palejwala SK, Sheen WA, Walter CM, et al. Minimally invasive lateral transpsoas interbody fusion using a stand-alone construct for the treatment of adjacent segment disease of the lumbar spine: review of the literature and report of three cases. Clin Neurol Neurosurg 2014;124:90-6. [Crossref] [PubMed]
- von Keudell A, Alimi M, Gebhard H, et al. Adult Degenerative Scoliosis with Spinal Stenosis Treated with Stand-Alone Cage via an Extreme Lateral Transpsoas Approach; a Case Report and Literature Review. Arch Bone Jt Surg 2015;3:124-9. [PubMed]
- Kepler CK, Bogner EA, Herzog RJ, et al. Anatomy of the psoas muscle and lumbar plexus with respect to the surgical approach for lateral transpsoas interbody fusion. Eur Spine J 2011;20:550-6. [Crossref] [PubMed]
- Hijji FY, Narain AS, Bohl DD, et al. Lateral lumbar interbody fusion: a systematic review of complication rates. Spine J 2017;17:1412-9. [Crossref] [PubMed]
- Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J 2014;14:749-58. [Crossref] [PubMed]
- Pumberger M, Hughes AP, Huang RR, et al. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J 2012;21:1192-9. [Crossref] [PubMed]
- Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 2011;15:11-8. [Crossref] [PubMed]
- Houten JK, Alexandre LC, Nasser R, et al. Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine 2011;15:280-4. [Crossref] [PubMed]
- Lee YP, Regev GJ, Chan J, et al. Evaluation of hip flexion strength following lateral lumbar interbody fusion. Spine J 2013;13:1259-62. [Crossref] [PubMed]
- Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine 2011;14:31-7. [Crossref] [PubMed]
- Cahill KS, Martinez JL, Wang MY, et al. Motor nerve injuries following the minimally invasive lateral transpsoas approach. J Neurosurg Spine 2012;17:227-31. [Crossref] [PubMed]
- Lykissas MG, Aichmair A, Sama AA, et al. Nerve injury and recovery after lateral lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a cohort-controlled study. Spine J 2014;14:217-24. [Crossref] [PubMed]
- Moro T, Kikuchi S, Konno S, et al. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine (Phila Pa 1976) 2003;28:423-8; discussion 427-8. [Crossref] [PubMed]
- Benglis DM, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine 2009;10:139-44. [Crossref] [PubMed]
- Park DK, Lee MJ, Lin EL, et al. The relationship of intrapsoas nerves during a transpsoas approach to the lumbar spine: anatomic study. J Spinal Disord Tech 2010;23:223-8. [Crossref] [PubMed]
- Uribe JS, Arredondo N, Dakwar E, et al. Defining the safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: an anatomical study. J Neurosurg Spine 2010;13:260-6. [Crossref] [PubMed]
- Knight RQ, Schwaegler P, Hanscom D, et al. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech 2009;22:34-7. [Crossref] [PubMed]
- Parikh K, Tomasino A, Knopman J, et al. Operative results and learning curve: microscope-assisted tubular microsurgery for 1- and 2-level discectomies and laminectomies. Neurosurg Focus 2008;25:E14. [Crossref] [PubMed]
- Sadrameli SS, Chu JK, Chan TM, et al. Minimally Invasive Tubular Tethered Cord Release in the Pediatric Population. World Neurosurg 2019;128:e912-e917. [Crossref] [PubMed]
- Sharif S, Afsar A. Learning Curve and Minimally Invasive Spine Surgery. World Neurosurg 2018;119:472-8. [Crossref] [PubMed]


