Complete resolution of chronic pain, sensory impairment, and motor dysfunction following percutaneous transforaminal endoscopic decompression in a failed back surgery syndrome patient—a case report
Introduction
Failed back surgery syndrome (FBSS)—when patients who have undergone laminectomy and decompression with or without instrumentation/fusion, suffer from persistent radicular and/or low back pain—represents a management challenge. Traditional treatment options range from conservative management including medications, physical therapy, and spinal injections (epidural, facet injections), to spinal cord stimulator implantation, but provide inconsistent pain relief and limited functional recovery. Transforaminal endoscopic decompression is a minimally invasive way to treat FBSS that has demonstrated patient satisfaction and improvement in pain and quality of life scores. However, prompt resolution of motor and sensory deficits has typically not been described. We present a case of percutaneous transforaminal endoscopic decompression resulting in full resolution of chronic low back and radicular pain, as well as immediate resolution of both sensory and motor dysfunction in a FBSS patient. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-586).
Case presentation
The patient is a 48-year-old female with chronic low back pain and lumbar radiculopathy since a motor vehicle collision (MVC) in 1991, worsened by a slip and fall in 2009. After failing conservative treatment, she underwent decompressive left hemi-laminectomy (L4/L5) in 2013. She developed FBSS with lumbar radiculopathy and functional left lower extremity weakness including foot drop which was not present preoperatively; thus, since her surgery, she had been bound to assistive devices or her wheelchair with very limited mobility and severe pain.
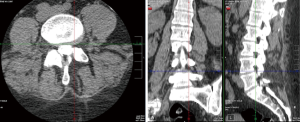
After failing more conservative treatments from 2013 to 2018, such as medications (50 mcg/hr fentanyl patch, tapentadol 75 mg q8 PRN, Percocet 10 mg-325 mg q8 PRN, gabapentin 300 mg TID, methocarbamol 750 mg QD, duloxetine 30 mg QD), physical therapy, epidural and facet injections, and being told that she was no longer a good surgical candidate due to her complex clinical picture and multiple comorbidities, she presented to our comprehensive spine clinic in September 2018. At the time of presentation, she was wheelchair bound with 7/10–10/10 pain, and lower left extremity 4/5 strength except for left ankle and great toe dorsiflexors (3/5 strength). The patient had an implanted non-MRI-compatible bladder stimulator for overactive bladder, thus a CT scan of the lumbar spine was performed, which showed only post-surgical changes (Figure 1). After discussing options, she elected to proceed with a caudal epidural steroid injection in October 2018 with attempted catheter adhesiolysis (3 mL of hyaluronidase and 80 mg depomedrol), from which she experienced good pain relief but only limited functional improvement for two months.
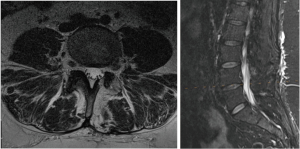
However, with the waning of analgesic effect of the caudal epidural steroid injection, the patient experienced worsening rebound pain, which necessitated hospital admission in January 2019. Her bladder stimulator was then removed in order for her to receive appropriate imaging. Magnetic resonance imaging (MRI) of the spine showed the expected postsurgical changes of a left side hemilaminectomy, as well as a left intraforaminal disc osteophyte at L4/5, mild L4/5 facet osteoarthritis, mild central canal stenosis and mild left intervertebral foramen stenosis at L4/5. Importantly, T2 signal changes at her previous surgical site surrounding the transiting left L5 nerve root was apparent (Figure 2) signifying likely epidural fibrosis/scarring.
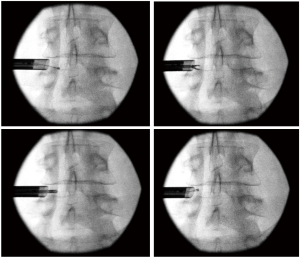
After two more caudal epidural steroid injections with catheter adhesiolysis were performed in February 2019 and August 2019 with diminishing effectiveness, the decision was made to perform percutaneous left lumbar L4/5 transforaminal endoscopic decompression (Figure 3). During the surgical procedure in January 2020, the transiting left L5 nerve root was visualized encased in thick scar tissue. This scar tissue was carefully dissected from around the nerve, until adequate decompression was confirmed by visualizing the nerve moving freely in the epidural space (Figure 4). Special care was taken to ensure the axilla between the exiting L4 and traversing L5 nerve roots, also known as the hidden zone of Macnab, was adequately decompressed of the residual foraminal disc and epidural scar. The procedure was performed primarily under local anesthesia while the patient was mildly sedated with remifentanil and dexmedetomidine infusions, so that communication was maintained. When adequate decompression was visually achieved, the patient was asked to move her left lower extremity; she was able to do so without any pain, paresthesia, or weakness for the first time in 7 years. The patient tolerated the procedure well without complication. She walked out of the post anesthesia care unit without any assistive devices, and without any pain.
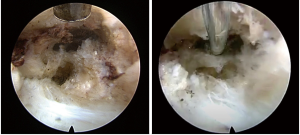
At her follow-up visit on the fourth postoperative day in January 2020, the patient stated she had complete resolution of her low back and radicular pain, as well as complete resolution of her left lower extremity weakness, numbness and foot drop; she had decreased her preoperative pain medication by over 50% (25 mcg/hr fentanyl patch and fewer opioid PRNs) and was no longer using any assistive devices. The patient has not had another follow-up appointment in the pain clinic since her procedure (Figure 5).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
FBSS, or postsurgical spine syndrome (1), is diagnosed when a patient has persistent back pain, with or without radicular symptoms, despite one or more surgical interventions. There is a 60–85% lifetime prevalence of low back pain (2), and the number of lower back surgeries performed is increasing; the number of primary lumbar fusions alone increased 170.7% (from 77,682 to 210,407) between 1998 and 2009 (3). The rates of failure of lumbar spine surgery are widely reported at 10–40% (4,5). When compared to those with osteoarthritis, rheumatoid arthritis, complex regional pain syndrome, and fibromyalgia, FBSS patients have higher pain levels and worse quality of life (6). Thus, FBSS is a significant problem affecting an increasingly large number of people.
The etiology of FBSS is multifactorial and can be divided into preoperative, operative, and post-operative factors. Pre-operative factors range from psychological factors to improper patient selection and revision surgery; operative factors include incomplete decompression and incorrect level surgery. Post-operative factors include recurrent disc herniation, adjacent segment disease, and nerve root entrapment syndrome (5). Epidural fibrosis causing nerve root entrapment is a significant problem, accounting for 20–36% of FBSS cases (2).
Conservative treatment options include pharmacologic therapy, physical therapy, and cognitive behavioral therapy; in addition, interventions such as epidural steroid injections or facet joint injections may be attempted. However, there is no consensus about the long-term benefit or best treatment algorithm (7-9).
Spinal cord stimulation (SCS) trials have shown that approximately 50% of patients have adequate pain relief at one year (9,10). However, SCS is not without its own challenges, including device complications, infection, and loss of therapeutic effect (9). In addition, while medication and neuromodulation are able to improve pain, they do not provide clinically significant improvement in subjective numbness and weakness; injections may provide transient relief, but may have decreasing efficacy with increasing number of treatments.
Revision open surgeries have been shown to have lower success rates as well (5), perhaps partially because the axilla, or hidden zone of Macnab, is almost impossible to reach through the traditional posterior approach to the spine (11). Transforaminal endoscopic surgeries are usually able to access this hidden zone. The initial approach is via Kambin’s triangle, a space defined anteriorly by the exiting nerve root, caudally by the end plate, posteriorly by the traversing nerve root and dural sac, and superiorly by the superior articular process (11,12).
When compared with open lumbar surgeries, endoscopic lumbar surgeries have many other benefits including less blood loss, less risk of infection, smaller incisions, and less surrounding soft tissue trauma. However, there is also a relatively steep learning curve with endoscopic lumbar decompression, with multiple studies demonstrating increased rate of more serious complications such as dural tear, infection, and hematoma when less experienced physicians perform the procedure (13).
Nonetheless, studies have demonstrated high patient satisfaction due to improvement of pain following endoscopic lumbar surgery. A study of 31 patients showed improved Visual Analog Scores (VAS) and Oswestry Disability Index (ODI) scores following endoscopic decompression for lumbar foraminal stenosis; the same study reported good clinical outcomes with 80% improvement in pain (14). Another study of 30 patients noted average pain improvement after transforaminal endoscopic surgery based on ODI and VAS scores, and also described a correlation between preoperative pain and patho-anatomy discovered within the hidden zone of Macnab (11). A study of 65 elderly patients with severe comorbidities presented percutaneous endoscopic ventral facetectomy as an option for lateral recess stenosis in these poor surgical candidates; each patient had an improved health related quality of life following endoscopic surgery, despite still experiencing back pain, attributed to general degenerative lumbar spine disease (15). Other studies have reported alleviation of pain and improvement in functional status following transforaminal endoscopic decompression among a wide variety of ages (16–86 years old) (16,17).
In a study of 6 physicians undergoing endoscopic surgery including discectomy for herniated nucleus pulposus or lateral recess stenosis, one patient was found to have a fragment compressing the S1 nerve root; this patient had weakness and numbness in addition to pain, which resolved over a 6-month time course postoperatively (17). Another case report discussed a 16-year-old long jump athlete who had a herniated nucleus pulposus and underwent transforaminal percutaneous endoscopic lumber discectomy; this patient had immediate full relief of pain, and improvement of lower extremity weakness over the next 4–8 weeks, after strength exercises (16). In another case report, a 40-year-old woman underwent total disc replacement, complicated by an acute foot drop three hours after surgery due to a bone fragment in the right L4-5 foramen; this fragment was removed by endoscopic decompression, and the patient’s strength was normal by the next day (18).
In all of the above-mentioned cases, patients had favorable outcomes (resolution or improvement of pain) following endoscopic surgery. However, improvement in sensation or motor function was rarely discussed. Of the three cases mentioning improvement in sensation and motor function, two patients regained function over a multiple-month time period; the other patient had an acute insult to her nerve root immediately postoperatively and regained her function quickly after removal of the offending bone fragment.
In this particular case, our patient had a 7-year history of weakness and numbness, which was immediately improved upon transforaminal endoscopic decompression of her tethered nerve root. In the case of nerve entrapment in scar tissue, we have shown that it is possible to fully free the nerve with return of function. Perhaps this full return of motor and sensory function wasn’t demonstrated in other studies due to incomplete freeing of the nerve, or due to different underlying pathology causing the patients’ pain and dysfunction.
Thus, there is an important role for transforaminal endoscopic decompression in FBSS; it may be a truly efficacious way of addressing epidural fibrosis causing nerve root entrapment. The traversing and exiting nerve roots can be directly visualized to see if they are encased in scar tissue, and bipolar radiofrequency cautery may be used to dissect away most or all of the surrounding scar tissue. In addition, areas difficult to reach in traditional spine surgery may be accessed and decompressed. In this way, the entire nerve can be freed resulting in not only pain resolution, but the potential for resolution of motor and sensory dysfunction as well. By keeping patients minimally sedated, they are able to provide real-time feedback in order to ensure the success of the procedure.
This particular case demonstrates the potential for this minimally invasive technique to have a profound impact on the function and quality of life in carefully chosen chronic FBSS patients. Not only did this particular patient’s pain completely resolve, but her sensory and motor deficits resolved as well.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-586
Peer Review File: Available at http://dx.doi.org/10.21037/jss-20-586
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-586). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ordia J, Vaisman J. Post-surgical spine syndrome. Surg Neurol Int 2011;2:132. [Crossref] [PubMed]
- Chan CW, Peng P. Failed back surgery syndrome. Pain Med 2011;12:577-606. [Crossref] [PubMed]
- Rajaee SS, Bae HW, Kanim LEA, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67-76. [Crossref] [PubMed]
- Thomson S. Failed back surgery syndrome - definition, epidemiology and demographics. Br J Pain 2013;7:56-9. [Crossref] [PubMed]
- Sebaaly A, Lahoud MJ, Rizkallah M, et al. Etiology, Evaluation, and Treatment of Failed Back Surgery Syndrome. Asian Spine J 2018;12:574-85. [Crossref] [PubMed]
- Thomson S, Jacques L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract 2009;9:206-15. [Crossref] [PubMed]
- Daniell JR, Osti OL. Failed Back Surgery Syndrome: A Review Article. Asian Spine J 2018;12:372-9. [Crossref] [PubMed]
- Ganty P, Sharma M. Failed back surgery syndrome: a suggested algorithm of care. Br J Pain 2012;6:153-61. [Crossref] [PubMed]
- Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132:179-88. [Crossref] [PubMed]
- Burchiel KJ, Anderson VC, Brown FD, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine (Phila Pa 1976) 1996;21:2786-94. [Crossref] [PubMed]
- Yeung A, Gore S. Endoscopic Foraminal Decompression for Failed Back Surgery Syndrome under Local Anesthesia. Int J Spine Surg 2014;8:22. [Crossref] [PubMed]
- Fanous AA, Tumialán LM, Wang MY. Kambin's triangle: definition and new classification schema. J Neurosurg Spine 2019;29:1-9. [PubMed]
- Butler AJ, Alam M, Wiley K, et al. Endoscopic Lumbar Surgery: The State of the Art in 2019. Neurospine 2019;16:15-23. [Crossref] [PubMed]
- Kim JE, Choi DJ, Park EJ. Clinical and Radiological Outcomes of Foraminal Decompression Using Unilateral Biportal Endoscopic Spine Surgery for Lumbar Foraminal Stenosis. Clin Orthop Surg 2018;10:439-47. [Crossref] [PubMed]
- Kapetanakis S, Gkantsinikoudis N, Thomaidis T, et al. The Role of Percutaneous Transforaminal Endoscopic Surgery in Lateral Recess Stenosis in Elderly Patients. Asian Spine J 2019;13:638-47. [Crossref] [PubMed]
- Sairyo K, Chikawa T, Nagamachi A. State-of-the-art transforaminal percutaneous endoscopic lumbar surgery under local anesthesia: Discectomy, foraminoplasty, and ventral facetectomy. J Orthop Sci 2018;23:229-36. [Crossref] [PubMed]
- Fujii Y, Yamashita K, Sugiura K, et al. Early return to activity after minimally invasive full endoscopic decompression surgery in medical doctors. J Spine Surg 2020;6:S294-9. [Crossref] [PubMed]
- Wagner R, Iprenburg M, Telfeian AE. Transforaminal endoscopic decompression of a postoperative dislocated bone fragment after a 2-level lumbar total disc replacement: case report. Neurosurg Focus 2016;40:E8. [Crossref] [PubMed]