Retrospective radiographic analysis of anterior lumbar fusion for high grade lumbar spondylolisthesis
Introduction
High-grade spondylolisthesis grades as Meyerding grade III or IV with more than 50% slippage of the vertebral body on the subjacent vertebral body. Most high-grade listheses are the result of isthmic spondylolisthesis, as complete disruption of the pars is typically necessary for the degree of anterior vertebral translation. Thus, as expected, high-grade spondylolisthesis most commonly occurs at the L5–S1 level (1).
Patients with high-grade L5–S1 spondylolisthesis commonly present with pain and neurological deficits. Studies show that the majority of patients with high-grade lumbar spondylolisthesis continue to remain symptomatic despite conservative treatment, necessitating eventual surgical treatment (2).
Because high-grade listheses account for a small minority of patients treated for spondylolisthesis, limited publications focus specifically on this topic. Controversy remains over optimal surgical treatment for high-grade isthmic spondylolisthesis (3).
Several articles describe posterior only approaches for lumbar fusion in patients with high-grade spondylolisthesis (4-8). In some series with posterior-only approaches, authors report high rates of pseudoarthrosis in the range of 16–39%, as well as postoperative neurological deficits ranging from 34–42% (5,7).
A limited number of publications describe combined anterior and posterior approaches for lumbar fusion in patients with high-grade spondylolisthesis, focusing primarily on the pediatric and adolescent populations (6,8,9). Overall, the patients treated with circumferential fusion techniques had satisfactory clinical and radiographic results, as determined by various standardized outcome scales. A paucity of data exists with respect to combined anterior and posterior lumbar fusion in the adult population.
In the current study, we describe a series of 5 consecutive adult patients treated with circumferential lumbar fusion for high-grade (>50% slippage) lumbar spondylolisthesis. The operative technique is described in detail with illustrations. Demographic, preoperative and postoperative radiographic parameters, and radiographic outcomes are reported. We discuss our preference and rationale for treating high-grade spondylolisthesis via a circumferential technique. We present the article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-597).
Methods
Patient population
This study used a retrospective analysis for a cohort of five consecutive adult patients treated with circumferential lumbar fusion procedures were identified from a quality database in an academic spine practice. Inclusion criteria included: age 18 years and older, high-grade spondylolisthesis (Meyerding grade ≥3) at L5–S1, and preoperative and postoperative clinical and radiographic records. All recorded, qualifying procedures within the practice from 2017 to 2019 were included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Mayo Clinic Hospital as IRB exempt with an IRB identifier 20-002916. Consent waiver granted due to de-identified, retrospective chart review.
Preoperative radiographic assessment
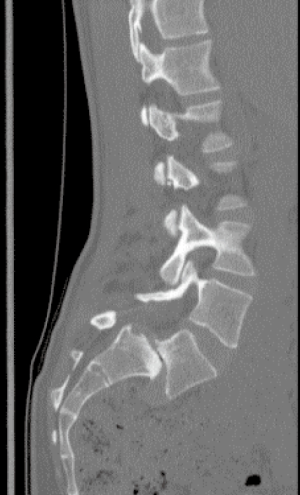
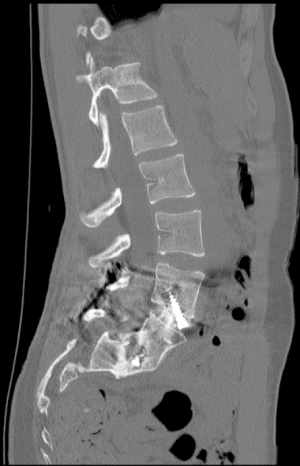
Prior to surgery, each patient underwent 36-inch scoliosis X-rays, dynamic flexion-extension lumbar X-rays, lumbar magnetic resonance imaging (MR) scans, and lumbar computerized tomography (CT) scans. Figure 1 demonstrates a sample preoperative lumbar computed tomography (CT) scan.
Surgical technique
After induction of general anesthesia with adequate vascular access, a large pliable bump (approximately 10 cm in diameter) is placed under the lumbar region. This maneuver increases lumbar lordosis and reduces the L5–S1 slippage in some cases. Blood pressure is constantly monitored by an arterial line in the radial position. Additionally, a pulse oximeter is used on the left hallux to provide monitoring of the perfusion to the left lower extremity throughout the case.
An infraumbilical midline incision approximately 10 cm in length is carried through the subcutaneous tissues to the level of the fascia. The subcutaneous fat is elevated from the fascia towards the left side of the linea alba for a length of approximately 5 cm. The fascia is incised 3 cm lateral and parallel to the linea alba. The fascial incision is extended proximally and distally to facilitate exposure to the retroperitoneum. The rectus muscle is elevated and moved laterally with the aid of a large handheld Richardson retractor (Medline Industries, Mundelein, IL). The epigastric vessels are retracted or ligated as necessary before being swept laterally with the rectus muscle. The peritoneum is swept medially and not violated. The retroperitoneal fat is then encountered and blunt dissection was carried down to the level of the psoas muscle. At this point in the dissection an Omni retractor (Omni-Tract Surgical, St. Paul, MN) with multiple renal vein retractors (usually 4–5) maintains the exposure. The left ureter is identified and protected as well as iliac artery and vein. Dissection is continued on the L5–S1 disk space until the middle sacral vein and artery are identified. These structures are ligated and transected. The L5–S1 disk space is cleared of soft tissue. Attention is then turned to the L4–L5 disk space. The left iliac vessels are usually mobilized medially to provide adequate exposure with care taken to ligate any lateral branches—namely iliolumbar vein—which may tear during mobilization. The pulse oximeter is also monitored for any change in perfusion to the left hallux, which could indicate significant compression of the arterial supply to the lower extremity. Appropriate exposure is confirmed with fluoroscopic guidance in the anterior and lateral positions. Exposure is maintained with renal vein retractors. The renal vein retractors are moved numerous times during the dissection to provide adequate visualization of structures, reduce prolonged venous compression and aid in blunt dissection.
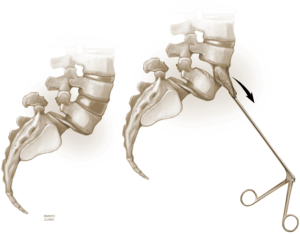
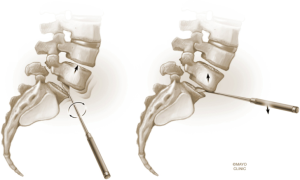
The L4–5 discectomy is performed first (Figure 2). Performing the L4–5 discectomy first allows for greater mobility when restoring the disk height and reducing the spondylolisthesis at the L5–S1 level. After performing a discectomy at the L4-5 interspace, the retractors are repositioned over the L5–S1 interspace. An annulotomy is performed using a 15 blade. We then use a mallet to gently tamp the Cobb instrument into the disk space. This step was performed with fluoroscopic guidance to ensure the endplates are not violated. The Cobb instrument is gently rocked clockwise and counter-clockwise to mobilize the disk space (Figure 3). We then use a series of trials to sequentially expand the disk height. As the disk height is restored, the spondylolisthesis is typically partially reduced. Final removal of disk material and endplate preparation is completed using standard technique (Figure 4). In the last step, the interbody graft is inserted. We use implants with integrated screws (Sovereign, Medtronic, Minneapolis, MN). The integrated screws are placed in both the cephalad and caudal directions. Alternatively, integrated screws may only be placed into the cephalad or caudal vertebral bodies. We have found that the posterior pedicle screws will overpower the smaller, anterior integrated screws. Consequently, additional reduction of spondylolisthesis is possible when posterior pedicle screws are placed, even if the integrated screws are placed into both adjacent vertebral bodies. The interbody spacer is then placed into the L4–5 interspace in the same fashion.
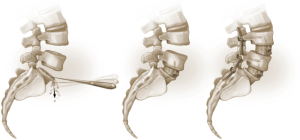
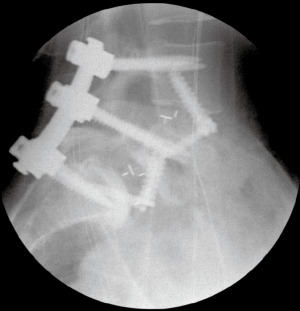
The surgical bed is inspected for hemostasis. Liposomal bupivacaine (Exparel® Pacira, San Diego) is then infiltrated into the lateral abdominal wall, anterior fascia, and subcutaneous tissues. The abdominal contents are returned to their normal anatomic positions and the fascia is closed. The subcutaneous fat is re-approximated and the skin is closed with subcuticular running suture. Good perfusion to the lower extremities is verified. The patient is then repositioned in the prone position. Posterior percutaneous pedicle screw and rod fixation is placed from L4–S1 through Wiltse incisions with O-arm® navigation. Additional reduction of the L5–S1 anterolisthesis is obtained by reducing the L5 screws to the rod (Figures 4,5).
Clinical and radiographic assessment
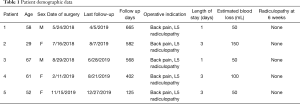
Medical and operative records were reviewed, and extracted data included: demographics, operative indication, length of stay, estimated blood loss, presence of radiculopathy at 6 weeks, and length of follow up. At last follow up, the Modified Macnab Criteria (10) were used to assess patient satisfaction with surgery.
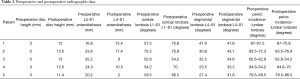
Four of the five patients underwent standing lumbar radiographs preoperatively and at regular follow-up intervals (2 weeks, 3 months and 12 months). One patient was unable to complete the 12-month follow-up X-rays. The radiographs were used to measure posterior disk height and millimeters of anterolisthesis at L5–S1 at the following time points: prior to surgery and at the final radiographic follow up. Segmental lordosis (L4–S1), total lumbar lordosis, and pelvic incidence—lumbar lordosis angle (PI-LL) mismatch were also measured prior to surgery and at last follow up.
Fusion was also assessed on the final radiographs or CT scan. Fusion was determined if there was (I) evidence of bridging trabecular bone across the disk space and (II) no sign of hardware loosening. Figure 6 demonstrates robust L4–S1 with bridging bone across interspaces. All radiographic measurements were the averaged measurements from 2 fellowship-trained spine surgeons. There was uniform agreement on spinal fusion in each case.
Statistical analysis
Descriptive statistics are reported as mean (range).
Results
Patients included 3/5 females and 2/5 males with mean age 57 years (range, 29–67 years). Presenting symptoms for all patients included intractable low back pain and L5 radicular symptoms.
Preoperative posterior disk height at L5–S1 for all patients was 0 mm. Mean preoperative L5–S1 spondylolisthesis at each level was 22.0 mm (range, 16.8–25.2 mm). Mean preoperative L4–S1 lordosis was 31.2° (range, 23.5°–41.9°), and mean preoperative L1–S1 lordosis was 59.1° (range, 52.9°–72.3°). Mean preoperative LL to PI mismatch was 16.4°.
Estimated blood loss was 80 cc (range, 50–100 cc). There were no intraoperative adverse events. Mean length of stay was 2.2 days (range, 1–3 days). There were no mortalities and no postoperative complications or reoperations to date of this manuscript. Six weeks following surgery all patients reported resolution of their preoperative L5 radicular symptoms.
Mean follow up was 468.4 days (range, 125–665 days). At last follow up, patient satisfaction, according to Modified Macnab Criteria, was excellent in 4/5 patients and good in 1/5 patient. Clinical and radiographic data listed in Tables 1 and 2. In the 4/5 patients with greater than 1-year radiographic follow-up, fusion rate was 100% on CT imaging. The last patient was unable to follow up after 4-month imaging. Mean increase in posterior disk height was 12.5 mm (range, 11.4–13.5 mm). Mean reduction in spondylolisthesis was 58.7% (range, 20.2–100%). Mean segmental (L4–S1) and overall (L1–S1) lumbar lordosis increased by 23.6% (range, 6.5–41.7%) and 16.6% (2.5–31.5%), respectively. Following surgery LL-PI mismatch decreased from a mean of 16.4° to 10.2°.
Full table
Full table
Discussion
High-grade lumbosacral spondylolisthesis results in debilitating pain and neurological symptoms. Durable treatment often requires surgery. Optimal surgical treatment remains controversial despite numerous described approaches. When an anterior approach is viable, we prefer a circumferential fusion technique for this patient population.
Numerous studies show the ALIF technique can effectively restore sagittal plane spinal parameters including segmental lumbar lordosis (SL), overall lumbar lordosis (LL), sagittal vertical axis (SVA), and PI-LL mismatch (11-13). When compared to posterior-only approaches, several reports find the ALIF procedure to have superior correction of sagittal plane parameters (12,14-17). Each of our patients had improvements in their sagittal measurements. In some cases, the improvement in lordosis was less than expected, likely because the posterior margin of the L5–S1 interbody graft, in most cases, was deliberately positioned near the posterior margin of the L5 vertebral body in order to maximize posterior disk and foraminal height. Furthermore, less lordotic implants were used to allow greater foraminal height restoration and indirect neural decompression.
ALIF provides excellent disk height restoration and indirect foraminal decompression (18-20). Due to large graft size and apophyseal ring coverage, ALIF procedures also have low rates of subsidence and resultant loss of the intraoperatively restored disk height (21).
During ALIF surgery, there is typically significant reduction of the spondylolisthesis when slippage is present (18,19). In a series of patients with grade 2 spondylolisthesis, Xu et al. found that ALIF often allowed for complete correction of spondylolisthesis (13).
Although disk height restoration and spondylolisthesis reduction are desirable, this is not always necessary for good patient outcome. Remes et al. described 67 patients that had undergone lumbar fusion for high-grade spondylolisthesis (9). With delayed follow up (mean 17.3 years), 28% of patients had foraminal stenosis with nerve root compression. No patients had radiculopathy symptoms. In addition, Oikonomidis et al. did not find that reduction of low grade spondylolisthesis was necessary for good patient outcome (22). In many cases, fixation and elimination of dynamic nerve compression can alleviate radiculopathy symptoms.
In this series, we found that posterior disk height at L5–S1 increased by an average of 12.5 mm, and spondylolisthesis was reduced by 58.7%. Our ALIF technique allows for stabilization of the lumbosacral segment with simultaneous, indirect decompression of the L5 nerve roots. We find that removal of the L4–5 disk prior to the L5–S1 ALIF facilitates graft insertion and height restoration at the L5–S1 level. We also surmise that gentle surgical technique and proper graft sizing is necessary to avoid excessive manipulation or stretching of the nerve roots.
No patients in this series experienced postoperative radicular symptoms. Some studies describing posterior only approaches for surgical treatment of high-grade spondylolisthesis report high rates of postoperative neurological deficits. In a study by Moreau et al., 50 adolescent and young adult patients treated for high-grade spondylolisthesis with posterior only approach, and 34% had temporary postoperative radicular deficit (7). In another study, 5/12 pediatric patients treated with posterior-only approaches for high-grade spondylolisthesis had temporary L5 or S1 radicular deficits following surgery (5). ALIF allows for simultaneous enlargement of the neural foramina in the horizontal plane—by way of reduction—and the vertical plane, via disc height elevation. We have found this to be an effective strategy to mitigate postoperative radicular deficits in the high-grade spondylolisthesis population.
The anterior approach for lumbar fusion allows for a large annulotomy and direct visualization of the disk space. Consequently, the surgeon can perform a thorough discectomy, prepare the endplates meticulously, and insert a large implant containing graft material. In general, ALIF has been associated with very high fusion rates (19-21). Some authors find ALIF to have superior fusion rates compared to posterior-only lumbar fusion approaches. Molinari et al. (23) describe surgical fusion for 31 pediatric patients with Meyerding Grade 3 or 4 isthmic dysplastic spondylolisthesis. The 18 patients treated only with posterior instrumentation (no anterior column support) had a 39% pseudoarthrosis rate. The 19 patients that had a circumferential fusion including anterior support with autogenous tricortical iliac crest graft had a 100% fusion rate. Circumferential lumbosacral fusion with ALIF allows for a robust osseous fusion which is necessary for best surgical outcome.
When ALIF is performed with good surgical technique, the surgeon can expect high fusion rates, even in cases of high-grade spondylolisthesis. This obviates the need for posterior exposure and arthrodesis. Posterior instrumentation can be placed percutaneously which results in less muscle trauma and subsequent degeneration. This is advantageous because open posterior lumbar surgery is associated with decreased spinal mobility and trunk strength (9).
ALIF is shown to be associated with significant improvements in patient-reported outcome measures (PROM) in spondylolisthesis patients (11-13). In one study involving low-grade spondylolisthesis patients, Chandra et al. (24) noted the ALIF patients had faster back pain relief than patients who had a posterolateral fusion only. In another study involving pediatric and adolescent patients with high-grade spondylolisthesis, Helenius et al. compared 26 patients treated with a circumferential fusion to 44 patients treated with anterior only or posterolateral only fusion techniques (6). Long-term follow up (mean 17.2 years) revealed that the circumferential fusion group had significantly better SRS total scores, better values for pain and function from back condition domains, and Oswestry Disability Index (ODI) scores compared to the anterior or posterior only fusion groups. The patients in the present series had average follow up of 468 days. All patients reported immediate and complete resolution of L5 radicular symptoms. All patients reported minimal or no back pain at final follow up.
In properly selected patients with high-grade lumbosacral spondylolisthesis, circumferential fusion with ALIF is a powerful technique that can decompress the neural elements, improve sagittal plane measurements, lead to a solid arthrodesis, and yield excellent patient outcomes.
This study has several limitations. High grade spondylolisthesis at L5–S1 is less commonly encountered in adults. Therefore, we have a limited sample size. We did not encounter any surgical complications in this limited group, and we are not able to comment on surgical risks. Surgical complications are expected in a larger patient sample. Typical complications associated with the anterior lumbar approach are approach related, including vascular, viscous, urological as well as neurological injury in the form of ventral CSF leak, sympathetic chain dysfunction as well as neurapraxia.
Conclusions
ALIF with posterior percutaneous instrumentation is a safe and effective treatment for high-grade lumbosacral spondylolisthesis in properly selected adults. Technical nuances described in this manuscript facilitate the procedure. This technique improves lumbar sagittal parameters and substantially reduces spondylolisthesis. The indirect neural decompression from simultaneous disk height restoration and spondylolisthesis reduction may be associated with lower neurological injury rate compared to posterior-only spinal fusion surgery. Future prospective study is needed to validate this hypothesis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-597
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-597
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-597). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Mayo Clinic Hospital as IRB exempt with an IRB identifier 20-002916. Consent waiver granted due to de-identified, retrospective chart review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beck AW, Simpson AK. High-Grade Lumbar Spondylolisthesis. Neurosurg Clin N Am 2019;30:291-8. [Crossref] [PubMed]
- Harris IE, Weinstein SL. Long-term follow-up of patients with Grade-III and IV spondylolisthesis. Treatment with and without posterior fusion. J Bone Joint Surg Am 1987;69:960-9. [Crossref] [PubMed]
- Goyal N, Wimberley DW, Hyatt A, et al. Radiographic and clinical outcomes after instrumented reduction and transforaminal lumbar interbody fusion of mid and high-grade isthmic spondylolisthesis. J Spinal Disord Tech 2009;22:321-7. [Crossref] [PubMed]
- Rajakumar DV, Hari A, Krishna M, et al. Complete anatomic reduction and monosegmental fusion for lumbar spondylolisthesis of Grade II and higher: use of the minimally invasive “rocking” technique. Neurosurg Focus 2017;43:E12. [Crossref] [PubMed]
- Bouyer B, Bachy M, Courvoisier A, et al. High-grade lumbosacral spondylolisthesis reduction and fusion in children using transsacral rod fixation. Childs Nerv Syst 2014;30:505-13. [Crossref] [PubMed]
- Helenius I, Lamberg T, Osterman K, et al. Posterolateral, anterior, or circumferential fusion in situ for high-grade spondylolisthesis in young patients: a long-term evaluation using the Scoliosis Research Society questionnaire. Spine (Phila Pa 1976) 2006;31:190-6. [Crossref] [PubMed]
- Moreau S, Lonjon G, Guigui P, et al. Reduction and fusion in high-grade L5-S1 spondylolisthesis by a single posterior approach. Results in 50 patients. Orthop Traumatol Surg Res 2016;102:233-7. [Crossref] [PubMed]
- Molinari RW, Bridwell KH, Lenke LG, et al. Anterior column support in surgery for high-grade, isthmic spondylolisthesis. Clin Orthop Relat Res 2002.109-20. [Crossref] [PubMed]
- Remes V, Lamberg T, Tervahartiala P, et al. Long-term outcome after posterolateral, anterior, and circumferential fusion for high-grade isthmic spondylolisthesis in children and adolescents: magnetic resonance imaging findings after average of 17-year follow-up. Spine (Phila Pa 1976) 2006;31:2491-9. [Crossref] [PubMed]
- Macnab I.. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am 1971;53:891-903. [Crossref] [PubMed]
- Hosseini P, Mundis GM, Eastlack RK, et al. Preliminary results of anterior lumbar interbody fusion, anterior column realignment for the treatment of sagittal malalignment. Neurosurg Focus 2017;43:E6. [Crossref] [PubMed]
- Tye EY, Tanenbaum JE, Alonso AS, et al. Circumferential fusion: a comparative analysis between anterior lumbar interbody fusion with posterior pedicle screw fixation and transforaminal lumbar interbody fusion for L5–S1 isthmic spondylolisthesis. Spine J 2018;18:464-71. [Crossref] [PubMed]
- Xu DS, Bach K, Uribe JS. Minimally invasive anterior and lateral transpsoas approaches for closed reduction of grade II spondylolisthesis: initial clinical and radiographic experience. Neurosurg Focus 2018;44:E4. [Crossref] [PubMed]
- Cho JY, Goh TS, Son SM, et al. Comparison of Anterior Approach and Posterior Approach to Instrumented Interbody Fusion for Spondylolisthesis: A Meta-analysis. World Neurosurg 2019;129:e286-93. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Arthur AS, Abecassis IJ, Abi-Aad KR, et al. Vascular. Oper Neurosurg (Hagerstown) 2019;17:S76-118. [Crossref] [PubMed]
- Ahlquist S, Park HY, Gatto J, et al. Does approach matter? A comparative radiographic analysis of spinopelvic parameters in single-level lumbar fusion. Spine J 2018;18:1999-2008. [Crossref] [PubMed]
- Rao PJ, Ghent F, Phan K, et al. Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci 2015;22:1619-24. [Crossref] [PubMed]
- Lammli J, Whitaker MC, Moskowitz A, et al. Stand-alone anterior lumbar interbody fusion for degenerative disc disease of the lumbar spine: results with a 2-year follow-up. Spine (Phila Pa 1976) 2014;39:E894-901. [Crossref] [PubMed]
- Riouallon G, Lachaniette CHF, Poignard A, et al. Outcomes of anterior lumbar interbody fusion in low-grade isthmic spondylolisthesis in adults: a continuous series of 65 cases with an average follow-up of 6.6 years. Orthop Traumatol Surg Res 2013;99:155-61. [Crossref] [PubMed]
- Rao PJ, Phan K, Giang G, et al. Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study. J spine Surg 2017;3:168-75. [Crossref] [PubMed]
- Oikonomidis S, Meyer C, Scheyerer MJ, et al. Lumbar spinal fusion of low-grade degenerative spondylolisthesis (Meyerding grade I and II): Do reduction and correction of the radiological sagittal parameters correlate with better clinical outcome? Arch Orthop Trauma Surg 2020;140:1155-62. [Crossref] [PubMed]
- Molinari RW, Bridwell KH, Lenke LG, et al. Complications in the surgical treatment of pediatric high-grade, isthmic dysplastic spondylolisthesis. A comparison of three surgical approaches. Spine (Phila Pa 1976) 1999;24:1701-11. [Crossref] [PubMed]
- Chandra V, Singh RK. Anterior lumbar inter-body fusion with instrumentation compared with posterolateral fusion for low grade isthmic-spondylolisthesis. Acta Orthop Belg 2016;82:23-30. [PubMed]