
Implications of sagittal alignment and complication profile with stand-alone anterior lumbar interbody fusion versus anterior posterior lumbar fusion
Introduction
Anterior lumbar interbody fusion (ALIF) is commonly utilized to treat a range of spine pathologies such as degenerative disk disease (DDD) and spondylolisthesis (1,2). Over the past few decades, the technique has been refined to improve outcomes and reduce the risk of complications (1). Anterior posterior lumbar fusion (APF) involves supplementing the standard ALIF technique with posterior spinal instrumentation and fusion for increased stability, optimizing the chances of fusion and clinical success (3,4).
Through the stand-alone ALIF technique (ST-ALIF), the posterior structures may be spared while still achieving indirect decompression, expansion of the disk space, and restoration of lumbar lordosis (1,5,6). This anterior approach to the lumbar spine allows for access to the anterior longitudinal ligament, intervertebral disc, and anterior column for enhanced fusion rates and potential sagittal plane correction (1,7). In addition, ST-ALIF has been shown to require less operating time, and result in decreased intraoperative complications, lower blood loss, and reduced length of stay (8,9).
The APF technique seeks to address problems related to initial interbody stability and postoperative implant migration observed in some series of ST-ALIF procedures (10). APF offers increased biomechanical stability, which is thought to enhance the likelihood of bony fusion when compared to other lumbar spinal fusion techniques (3,11). However, APF has been associated with increased operative time, blood loss, length of stay, and complications when compared to other fusion techniques, namely transforaminal lumbar interbody fusion (TLIF) (12,13). Still, ST-ALIF and APF remain highly useful options to treat degenerative diseases of the spine given their favorable clinical outcomes (3,10).
The literature regarding ST-ALIF and APF has demonstrated satisfactory fusion rates and clinical success; however, the effect of these techiques on sagittal alignment and pelvic parameters, including SL, LL, and PI-LL mismatch has not yet been fully investigated. We hypothesize that ST-ALIF would have inferior radiographic results and greater risk for complications post-operatively when compared to APF given prior literature showing lower fusion rates (14,15) and decreased biomechanical stability with standalone cages (11), both of which could theoretically result in less stable constructs more prone to implant-related surgical complications that simultaneously worsen radiographic outcomes. The purpose of this study was to directly compare the ability of ST-ALIF and APF to influence SL, LL, and PI-LL mismatch post-operatively, as well as determine the complication profile of these techniques. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-595).
Methods
Ninety-two patients underwent anterior lumbar interbody fusion at a single institution from June 2013 to December 2018. Data from this cohort were retrospectively reviewed for variables including age, gender, body mass index (BMI), Charlson Comorbidity Index (CCI), smoking status and pre-operative diagnosis and surgical data including the spinal level, implant lordosis, and use of Bone Morphogenetic Protein (BMP). Radiographic measurements were performed on pre-operative and post-operative radiographs of all cases, including segmental lordosis (SL), lumbar lordosis (LL), pelvic incidence (PI), and pelvic incidence-lumbar lordosis mismatch (PI-LL). X-rays were obtained immediately post-operatively, at 2 weeks, 3 months, 6 months, 1 year and annually thereafter. Minimum radiographic follow-up was 6 months to be included in the study. Radiographic measurements were acquired from the standing lateral lumbar spine X-ray at final follow up. Surgical complications were reviewed and compared including subsidence, implant failure, nonunion, recurrent spondylolisthesis, and revision surgery.
ALIFs were performed by 6 spine surgeons with abdominal approaches all being performed by a vascular surgeon. All cases were categorized into either the ST-ALIF or APF cohort. Fifty-seven of the 92 cases underwent ST-ALIF and 35 underwent APF. ST-ALIF constructs were comprised of either a standalone interbody cage with integrated locking screws or an interbody cage with anterior locking plate with locking screws. APF groups had the addition of posterior spinal instrumentation and fusion (PSIF). Eight of the 35 (22.9%) APF cases were staged procedures where the anterior and posterior fusions occurred on different days as opposed to the same day.
Subsidence was defined as a loss of disc height of greater than or equal to 2 mm. Subsidence of the cage was measured as the average intervertebral disc height of the anterior and posterior aspects of the disc normalized to the anteroposterior diameter of the superior vertebral body to correct for magnification differences in radiographs at later timepoints compared to that measurement immediately postoperative (16,17). Early subsidence was defined as occurring within the first 6 weeks after surgery and delayed subsidence was defined as occurring greater than or equal to 6 weeks postoperatively. Severe subsidence was defined as greater than 3 mm and mild was defined as less than or equal to 3 mm. Nonunion was diagnosed by coronal and axial thin-section CT scans in all cases. Types of implant failure included fracture and/or loosening of the screws or rods.
Statistical analysis
Chi-Square test of homogeneity, Fisher’s exact test, and independent sample t-test were performed in order to establish significant differences between groups. Ninety-five percent confidence intervals for risk difference (RD) were calculated to denote the magnitude of these differences. Comparisons between groups and within groups were deemed statistically significant at the P<0.05 threshold. There was no missing data from subjects included in this study or statistical analysis. There were no subjects lost to follow-up as these subjects were excluded from the study based on inclusion requirements above. Sensitivity analyses were not indicated by study design. Statistical analyses were performed using SPSS 25 software (IBM Corp., Armonk, NY, USA).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of the David Geffen School of Medicine at the University of California - Los Angeles (No. 18-000760) and individual consent for this retrospective analysis was waived.
Results
Ninety-two patients underwent anterior lumbar interbody fusion at a single institution and met criteria for inclusion in this study based on radiograohic and clinical follow-up. Based on institutional approval, subjects not meeting this requirement were not tallied nor provided for research purposes. As such, a flow diagram of inclusion is limited to include only the 92 cases above. Fifty-seven of the 92 cases underwent ST-ALIF and 35 underwent APF. There were no losses to follow-up nor missing data for analyses.
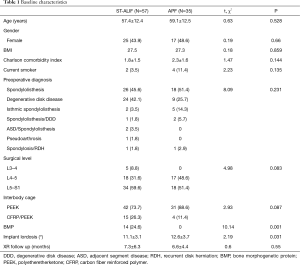
There were no significant demographic differences between the ST-ALIF and APF groups (Table 1). The average age was 58 years, with a similar gender distribution between groups. ALIF was most commonly indicated for spondylolisthesis, specifically at the L5-S1 level. The overall patient population demonstrated a moderate burden of health issues reflected in an average CCI of 2. Nearly 80% of patients utilized a polyetheretherketone (PEEK) cage, with the remaining receiving a combination carbon fiber reinforced polymer-PEEK cage. Average implant lordosis was significantly higher in the ST-ALIF group compared to the APF group by 1.5 degrees. Overall average implant lordosis for both groups was 11-12 degrees. BMP was used 14 times in the APF group but not in the ST-ALIF group (24.6% vs. 0%, P=0.001). Average radiographic follow up time for the ST-ALIF and APF cohorts was 7.3 and 6.6 months, respectively, which was not significantly different (P=0.550).
Full table
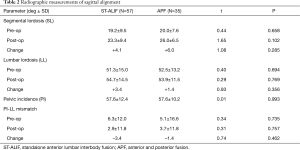
Pre-operative pelvic incidence (PI), segmental lordosis (SL), and lumbar lordosis (LL) were similar between the ST-ALIF and APF groups (Table 2). There was no statistical difference in SL between the ST-ALIF and APF groups despite higher implant lordosis in the ST-ALIF cohort. LL/PI-LL mismatch improvement was higher in the ST-ALIF group, but this did not reach statistical significance. Overall, changes in sagittal alignment were modest, and not significantly different between the two groups.
Full table
Complications were rare, but occurred more frequently in the ST-ALIF group, while there were no observed surgical complications in the APF group. Fusion rate was high in both groups, however the ST-ALIF group had 2 cases of nonunion, thus resulting in a 96.4% fusion rate compared to 100% in the APF group. The nonunions occurred in nonsmokers. Subsidence was the most frequent complication and was significantly more likely to occur in the ST-ALIF cohort versus the APF cohort (12.3% vs. 0%, RD 95% CI: 3.8–20.8%, P=0.042). Implant failure (5.3% vs. 0%) and recurrent spondylolisthesis (8.8% vs. 0%) were also more likely to occur in the ST-ALIF group, but this did not reach statistical significance (Table 3).

Full table
Eight patients had surgical complications in the ST-ALIF cohort. There were 7 cases of revision surgery, 6 of which were for subsidence and the other was for nonunion/implant failure. Revision surgery was posterior spinal instrumentation and fusion (PSIF) in these cases. A total of 71% of re-operations were within 90 days of the index surgery (Table 4).

Full table
Five of the 7 patients with post-operative subsidence had recurrent spondylolisthesis (71%) and all but one of those required revision surgery. The remaining patient was scheduled for surgery but had not received it at the time of the study. Ultimately, 86% of subsidence cases required revision surgery, and thus subsidence increased the odds of revision surgery by 6.92 (OR 95% CI: 1.13–42.5, P<0.001) (Table 4). Average subsidence was 4.10 mm (range, 2.10–8.00) and occurred most frequently on the inferior endplate (42.8%), followed by equal rates of superior endplate and both endplates (28.6%) (Table 4). The average time to detection of subsidence was 134 days (Range 14–468). Early subsidence occurred in 4 of 7 cases (57.1%) and severe subsidence in 3 of 7 cases (42.8%).
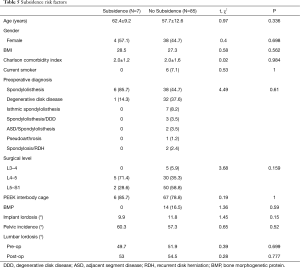
Several variables were different between the subsidence and non-subsidence groups, but none of them reached statistical significance (Table 5). Patients with subsidence had a non-significantly higher likelihood of older age (62.4 vs. 57.7 years), female gender (57.1% vs. 44.7%), higher BMI (28.5 vs. 27.3), L4-5 spinal disease (71.4% vs. 35.3%), spondylolisthesis as their pre-operative diagnosis (85.7% vs. 44.7%), no intraoperative BMP use (0% vs. 16.5%), lower implant lordosis (9.9° vs. 11.8°), and higher PI (60.3° vs. 57.3°).
Full table
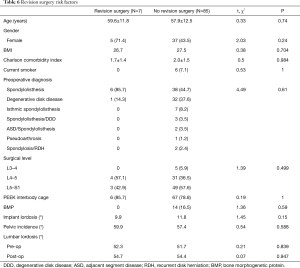
Given that revision surgery occurred in 6 out of 7 instances of subsidence, the subset of the ST-ALIF that required revision surgery had relatively similar trends to the subsidence group. Nonetheless, the revision surgery group had non-significantly higher likelihood of older age (59.6 vs. 57.9 years), female gender (71.4% vs. 43.5%), L4-5 spinal disease (71.4% vs. 35.3%), spondylolisthesis as their pre-operative diagnosis (85.7% vs. 44.7%), no intraoperative BMP use (0% vs. 16.5%), lower implant lordosis (9.9° vs. 11.8°), and higher pelvic incidence (59.9° vs. 57.4°) than the group that did not require revision surgery (Table 6). Multivariate analysis with logistic regression was not performed due to an inadequate sample size of subsidence and revision cases.
Full table
Discussion
ALIF is a commonly performed and effective method for addressing degenerative spinal pathology and attaining sagittal correction. ST-ALIF is an attractive option given the potential for decreased operative time, hospital length of stay, and blood loss in addition to avoiding the added morbidity of a second surgical procedure (8,9,12,18,19). While ST-ALIF has shown promising clinical outcomes (8,20-22). continued concerns remain over its stability and surgical complications. As a result, some spine surgeons add supplementary fixation posteriorly to enhance segmental stability. The APF strategy allows for a more stable construct with increased fusion rates (14) due to less movement at the operative level. APF has been shown in the literature to have good clinical outcomes (19,23), but has its own set of complications including an elevated long-term risk for adjacent segment disease (ASD) requiring re-operation (24). Thus, this study sought to directly compare ST-ALIF and APF in terms of radiographic outcomes and complications. This study found that radiographic outcomes were similar with fusion and sagittal alignment between the two techniques, but ST-ALIF was associated with greater rates of subsidence and revision surgery.
Surgical complications (nonunion, subsidence, implant failure)
In ST-ALIF there are rare reports of sacral (25) and L4 vertebral body (26) fractures, but the main concerns revolve around nonunion, subsidence and instrumentation failure. There is no consensus in the literature as to whether ST-ALIF or APF has better fusion rates (9,15) or clinical outcomes (8,19).
Nonunion
Our fusion rate for ST-ALIF of 96.4% was within the range of that reported in the literature (88–96%) (27-30), which may be influenced by the BMP usage in our ST-ALIF cohort (24.6%). McCarthy et al. compared ST-ALIF to APF in terms of fusion rates and found a significantly higher rate of fusion with ALIF with supplementary posterior pedicle screw fixation (15), correlating with the findings of our study where the APF cohort had a 100% fusion rate.
Subsidence
Subsidence is an important consideration as the loss of disc space height has the potential to adversely affect the sagittal correction and indirect decompression achieved by ALIF. Interbody cages have been used as an alternative to bone graft as they better resist disc space height loss, most recently with PEEK cages, given its similar modulus of elasticity to bone. Subsidence in ALIF has been reported anywhere from 2–16% (29-31) and higher in osteoporotic patients (32). In our ST-ALIF cohort we found a subsidence rate of 12.3%, which falls within the range reported in the literature. Our APF cohort did not have any cases of subsidence. There were no associations with early versus late and mild-moderate versus severe subsidence.
The timing of subsidence in the ALIF literature has been reported to occur within 15 days of surgery with femoral allograft (33) and within the first 3 months with iliac crest autografts (17) Timing to subsidence when utilizing an interbody cage has been reported at 2.75 months (range, 0.25–8 months) (16). In the present study, the average time to detection of subsidence was 4.5 months. However, 2 cases were detected within 14 days of surgery illustrating the highly variable nature of the timing of subsidence. It should be noted that the timing of subsidence detection is likely impacted by patient follow up, or lack thereof.
The literature is also variable on the most common location of subsidence in ALIF with some reporting it more frequently on the superior endplate (30) correlating with biomechanical studies (34), while others report subsidence occurs more commonly on both endplates simultaneously (16). Subsidence in this study most commonly occurred on the inferior endplate (43%), but also occurred at a substantial rate on the superior endplate as well as both endplates simultaneously.
Previous studies have questioned the clinical impact of subsidence (16,30), and while our study does not include patient-reported outcomes, subsidence appears to be clinically relevant in the present study because it was accompanied by recurrent spondylolisthesis and need for revision surgery at substantial rates (71% and 86%, respectively). This high rate of revision surgery in subsidence cases is also reflected in a study by Behrbalk et al. where 60% of subsidence cases failed to fuse and required revision surgery (31) High grade subsidence has previously been shown to be a predictor of need for revision surgery (35) in the ST-LLIF literautre, but the degree of subsidence in our study did not correlate with revision surgery, rather only the presence of subsidence.
There is no consensus in the literature as to what risk factors exist for subsidence. Some studies in the ST-ALIF literature have shown increased age (36), a spondylolisthesis diagnosis (31), and the location of cage positioning on the endplate (34) to be risk factors. Other studies are conflicted on whether increased lordosis (30,37) and BMI (31,38) pose an increased risk for subsidence. In the standalone-lateral lumbar interbody fusion literature, bone mineral density (39) and cage width (40) have been shown to affect subsidence rate. Our study mirrored some of the findings in the ST-ALIF literature in that those with subsidence trended toward increased age, BMI, and likelihood of a spondylolisthesis diagnosis, but these differences were not statistically significant.
Implant failure
Our study found 3 cases of implant failure (5.3%) in the ST-ALIF cohort, all of which required re-operation; this is a lower rate than has been reported in the literature (18.8%) (27). There were no instances of implant failure in the APF cohort, which perhaps may be due to posterior instrumentation being able to better tolerate physiologic loads.
Sagittal alignment/radiographic measurements
This study found no significant differences between the ST-ALIF and APF cohorts in terms of pre and post-operative radiographic parameters. There were small numerical differences in ΔSL (+1.9°) and ΔLL (−2.0°) in the APF cohort compared to the ST-ALIF cohort, but these findings were not statistically significant (P>0.29). Pre and post-operative PI and LL were also found to not be significantly different in the subsidence and non-subsidence groups. Overall, alterations in sagittal alignment were modest in both cohorts in this study and no radiographic parameters were found to be significantly different between the two cohorts.
Strengths
This is the first study to our knowledge that directly compares ST-ALIF versus APF in terms of both surgical complication profile and radiographic outcomes. It demonstrated that subsidence was clinically relevant in ST-ALIF as nearly all patients in this cohort required revision surgery. This study also uniquely provides data on additional complications such as nonunion, implant failure, recurrent spondylolisthesis as well as detailed information on subsidence extent, location, and timing all within the same cohort. This study removes confounding present in other studies by only including single level fusions and is more externally valid given the fact that multiple different surgeons performed the surgical procedures.
Limitations
Our study has several limitations. Namely, this is a retrospective comparative study subject to bias. While almost all of the baseline characteristics and treatment variables were not significantly different between groups, BMP and implant lordosis were significantly different between the ST-ALIF and APF cohorts. There is also possible indication bias in our study in that patients with presumed lower risk for complications may have been preferentially selected for ST-ALIF (although CCI was not significantly different between groups). Thus, our study may under-report the complication rate of ST-ALIF. Despite this possibility, APF resulted in significantly fewer complications with none overall. Despite increased external validity, potential differences in surgical technique amongst surgeons is a possible confounder. Other weaknesses of our study include the absence of bone mineral density measurements and patient reported outcome measures to correlate with surgical complications and radiographic outcomes. This study was also underpowered to detect specific risk factors for subsidence in sub-group analysis of the ST-ALIF cohort and follow up was too short to evaluate for the development of ASD in either cohort. Certainly, larger, randomized clinical trials would be necessary to affirm the findings described in the present study.
Conclusions
ST-ALIF was associated with significantly higher rates of subsidence and revision surgery as compared to APF in this study. Careful consideration should be given to patient selection, particulary to bone quality and risk factors for nonunion, when considering ST-ALIF. The potential for revision surgery and additional hospitalization time may offset any potential benefit present in avoiding same-day or staged posterior spinal instrumentation and fusion. Despite the greater risk of subsidence, sagittal alignment was not significantly affected in this study. Additional confounding risk factors for subsidence may exist concurrently in ST-ALIF patients, but these factors require a larger sample size to be adequately evaluated in order to improve patient selection. Further large scale, multi-center, randomized clinical trials may be necessary to investigate further.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-595
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-595
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-595). Dr. Park reports personal fees from Stryker, personal fees from Nuvasive, personal fees from Globus, personal fees from Seaspine, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of the David Geffen School of Medicine at the University of California - Los Angeles (No. 18-000760) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mobbs RJ, Loganathan A, Yeung V, et al. Indications for anterior lumbar interbody fusion. Orthop Surg 2013;5:153-63. [Crossref] [PubMed]
- Spiker WR, Goz V, Brodke DS. Lumbar Interbody Fusions for Degenerative Spondylolisthesis: Review of Techniques, Indications, and Outcomes. Global Spine J 2019;9:77-84. [Crossref] [PubMed]
- Faundez AA, Schwender JD, Safriel Y, et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J 2009;18:203-11. [Crossref] [PubMed]
- Kozak JA, O'Brien JP. Simultaneous combined anterior and posterior fusion. An independent analysis of a treatment for the disabled low-back pain patient. Spine (Phila Pa 1976) 1990;15:322-8. [Crossref] [PubMed]
- Amaral R, Ferreira R, Marchi L, Jensen R, Nogueira-Neto J, Pimenta L. Stand-alone anterior lumbar interbody fusion - complications and perioperative results. Rev Bras Ortop 2017;52:569-74. [Crossref] [PubMed]
- Dickerman RD, East JW, Winters K, et al. Anterior and posterior lumbar interbody fusion with percutaneous pedicle screws: comparison to muscle damage and minimally invasive techniques. Spine (Phila Pa 1976) 2009;34:E923-5. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Strube P, Hoff E, Hartwig T, et al. Stand-alone anterior versus anteroposterior lumbar interbody single-level fusion after a mean follow-up of 41 months. J Spinal Disord Tech 2012;25:362-9. [Crossref] [PubMed]
- Bozzio AE, Johnson CR, Fattor JA, et al. Stand-alone Anterior Lumbar Interbody, Transforaminal Lumbar Interbody, and Anterior/Posterior Fusion: Analysis of Fusion Outcomes and Costs. Orthopedics 2018;41:e655-e662. [Crossref] [PubMed]
- Zhang JD, Poffyn B, Sys G, et al. Are stand-alone cages sufficient for anterior lumbar interbody fusion? Orthop Surg 2012;4:11-4. [Crossref] [PubMed]
- Oxland TR, Lund T. Biomechanics of stand-alone cages and cages in combination with posterior fixation: a literature review. Eur Spine J 2000;9 Suppl 1:S95-101. [Crossref] [PubMed]
- Goz V, Weinreb JH, Schwab F, et al. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J 2014;14:2019-27. [Crossref] [PubMed]
- Villavicencio AT, Burneikiene S, Bulsara KR, et al. Perioperative complications in transforaminal lumbar interbody fusion versus anterior-posterior reconstruction for lumbar disc degeneration and instability. J Spinal Disord Tech 2006;19:92-7. [Crossref] [PubMed]
- Anjarwalla NK, Morcom RK, Fraser RD. Supplementary Stabilization With Anterior Lumbar Intervertebral Fusion–A Radiologic Review. Spine (Phila Pa 1976) 2006;31:1281-7. [Crossref] [PubMed]
- McCarthy MJH, Ng L, Vermeersch G, et al. A Radiological Comparison of Anterior Fusion Rates in Anterior Lumbar Interbody Fusion. Global Spine J 2012;2:195-206. [Crossref] [PubMed]
- Choi JY, Sung KH. Subsidence after anterior lumbar interbody fusion using paired Stand-alone rectangular cages. Eur Spine J 2006;15:16-22. [Crossref] [PubMed]
- Cheung KMC, Zhang YG, Lu DS, et al. Reduction of disc space distraction after anterior lumbar interbody fusion with autologous iliac crest graft. Spine (Phila Pa 1976) 2003;28:1385-9. [Crossref] [PubMed]
- Lammli J, Whitaker MC, Moskowitz A, et al. Stand-alone Anterior Lumbar Interbody Fusion for Degenerative Disc Disease of the Lumbar Spine: Results with 2 year follow up. Spine (Phila Pa 1976) 2014;39:E894-901. [Crossref] [PubMed]
- Kim JS, Kim DH, Lee SH, et al. Comparison Study of the Instrumented 360D Fusion with Instrumented Anterior Lumbar Interbody Fusion as a Surgical Procedure for Adult Low-Grade Isthmic Spondylolisthesis. World Neurosurg 2010;73:565-71. [Crossref] [PubMed]
- Udby PM, Bech-Azeddine R. Clinical outcome of ST-ALIF compared to posterior instrumentation for degenerative disc disease: A pilot study and a literature review. Clin Neurol Neurosurg 2015;133:64-9. [Crossref] [PubMed]
- Giang G, Mobbs R, Phan S, et al. Evaluating Outcomes of Stand alone Anterior Lumbar Interbody Fusion: A Systematic Review. World Neurosurg 2017;104:259-71. [Crossref] [PubMed]
- Rao PJ, Ghent F, Phan K, et al. Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci 2015;22:1619-24. [Crossref] [PubMed]
- Hinkley BS, Jaremko ME. Effects of 360-Degree Lumbar Fusion in a Workers’ Compensation Population. Spine (Phila Pa 1976) 1997;22:312-22. [Crossref] [PubMed]
- Maruenda JI, Barrios C, Garibo F, et al. Adjacent segment degeneration and revision surgery after 360D lumbar fusion: outcomes throughout 15 years of follow-up. Eur Spine J 2016;25:1550-7. [Crossref] [PubMed]
- Lastfogel JF, Altstadt TJ, Rodgers RB, et al. Sacral fractures following stand-alone L5–S1 anterior lumbar interbody fusion for isthmic spondylolisthesis. J Neurosurg Spine 2010;13:288-93. [Crossref] [PubMed]
- Kwon YK, Jang JH, Lee CD, et al. Fracture of the L-4 vertebral body after use of a Stand-Alone interbody fusion device in degenerative spondylolisthesis for anterior L3–4 fixation. J Neurosurg Spine 2014;20:653-6. [Crossref] [PubMed]
- Jaeger A, Giber D, Bastard C, et al. Risk factors of instrumentation failure and pseudarthrosis after stand-alone L5-S1 anterior lumbar interbody fusion: a retrospective cohort study. J Neurosurg Spine 2019;31:338-346. [PubMed]
- Manzur M, Virk SS, Jivanelli B, et al. The rate of fusion for stand-alone anterior lumbar interbody fusion: a systematic review. Spine J 2019;19:1294-301. [Crossref] [PubMed]
- Allain J, Delecrin J, Beaurain J, et al. Stand-Alone ALIF with integrated intracorporeal anchoring plates in the treatment of degenerative lumbar disc disease: a prospective study on 65 cases. Eur Spine J 2014;23:2136-43. [Crossref] [PubMed]
- Rao PJ, Phan K, Giang G, et al. Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study. J Spine Surg 2017;3:168-75. [Crossref] [PubMed]
- Behrbalk E, Uri O, Parks RM, et al. Fusion and subsidence rate of stand alone anterior lumbar interbody fusion using PEEK cage with recombinant human bone morphogenetic protein-2. Eur Spine J 2013;22:2869-75. [Crossref] [PubMed]
- Kim KH, Lee SH, Lee DY, et al. Anterior bone cement augmentation in anterior lumbar interbody fusion and percutaneous pedicle screw fixation in patients with osteoporosis. J Neurosurg Spine 2010;12:525-32. [Crossref] [PubMed]
- Kumar A, Kozak JA, Doherty BJ, et al. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine (Phila Pa 1976) 1993;18:2393-400. [Crossref] [PubMed]
- Grant JP, Oxland TR, Dvorak MF, et al. The effects of bone density and disc degeneration on the structural property distributions in the lower lumbar vertebral endplates. J Orthop Res 2002;20:1115-20. [Crossref] [PubMed]
- Tempel ZJ, McDowell MM, Panczykowski DM, et al. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg Spine 2018;28:50-6. [Crossref] [PubMed]
- Phan K, Ramachandran V, Tran T, et al. Impact of Elderly Age on Complications and Clinical Outcomes Following Anterior Lumbar Interbody Fusion Surgery. World Neurosurg 2017;105:503-9. [Crossref] [PubMed]
- Rahn KA, Shugart RM, Wylie MW, et al. The effect of lordosis, disc height change, subsidence, and transitional segment on Stand-Alone anterior lumbar interbody fusion using a nontapered threaded device. Am J Orthop (Belle Mead NJ) 2010;39:E124-9. [PubMed]
- Phan K, Rogers P, Rao PJ, et al. Influence of Obesity on Complications, Clinical Outcome, and Subsidence After Anterior Lumbar Interbody Fusion (ALIF): Prospective Observational Study. World Neurosurg 2017;107:334-41. [Crossref] [PubMed]
- Tempel ZJ, Gandhoke GS, Okonkwo DO, et al. Impaired bone mineral density as a predictor of graft subsidence following minimally invasive transpsoas lateral lumbar interbody fusion. Eur Spine J 2015;24 Suppl 3:414-9. [Crossref] [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-Alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8. [Crossref] [PubMed]


