
Factors important in bone union after posterior lumbar interbody fusion using the cortical bone trajectory technique
Introduction
Cortical bone trajectory (CBT), which was advocated by Santoni et al. in 2009, is a technique for posterior lumbar interbody fusion (PLIF) (1). The use of CBT has become widespread use because it is less invasive than other methods and it results in a solid fixation, as demonstrated by biomechanical studies (2-4).
The purpose of PLIF is to reduce neurogenic and lumbar pain due to neural irritation and intervertebral instability. Whether the intervertebral fusion status correlates with clinical outcomes is controversial (5-7). However, solid fusion may be an important factor in achieving successful PLIF because nonunion could lead to screw loosening, implant failure, cage retropulsion, and revision surgery (2,8,9). Gender, age, osteoporosis, smoking, rheumatism, and steroid use have been all reported as factors associated with nonunion after spinal fusion (7,9-14). Fujibayashi et al. also reported postoperative endplate cyst formation as a predictor of nonunion (15).
Sakaura et al. reported a better Japanese Orthopedic Association (JOA) score after CBT-PLIF than after conventional-PLIF and that CBT-PLIF tended to have a low bone union rate (16). Furthermore, although Kaito et al. reported an increased incidence of diffuse endplate cyst formation at three months after CBT-PLIF compared to conventional-PLIF, they also reported that the bone union rate at one year indicated no significant difference between conventional-PLIF and CBT-PLIF (17). However, to the best of our knowledge, the factors contributing to the bone union rate in CBT-PLIF are unclear. The aim of this study was to investigate radiographic factors that are important in bone union in CBT-PLIF. We present the following article/case in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-608).
Methods
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee of Murayama Medical Center (receipt ID: 12-10) and individual consent for this retrospective analysis was waived. Sixty-nine consecutive patients (50 males and 19 females) who were treated with “single” level CBT-PLIF from October 2011 to December 2016 were enrolled retrospectively in this study. The exclusion criteria were trauma, tumor, infection, and congenital disease. The mean age and standard deviation (SD) were 51.1±16.2 years (range, 24−81 years). The preoperative diagnoses were 29 cases of degenerative spondylolisthesis, 24 cases of lumbar central huge disc herniation, and 16 cases of degenerative disc disease. We followed all patients for at least 24 months (mean and SD 31.6±9.17 months).
Surgical procedure
We exposed the surgical field by a midline incision and a spinous process splitting approach and preserved the adjacent cranial facet joint. The entry point of the CBT screw was made by a 2 mm high-speed drill under fluoroscopic support, in accordance with the technique of Matsukawa et al. (18). The direction of the CBT screw was set at 25°cranial and 10° lateral. The holes were expanded in two steps, using probes of different diameters, and pedicle marker pins were placed into the holes. The decompression and interbody procedures were then performed. The screw paths were tapped to the same size as those of the screws to be used, to prevent starting point fracture. Lastly, screws were inserted and rods were connected under compressive force. The SOLERA Spinal System (Medtronic Sofamor Danek) and MATRIX Spine System (Depuy Synthes) were used in 67 cases and two cases (mean diameter 5.59±0.47 mm, mean length 33.0±3.32 mm). The PEEK cage was CAPSTONE (Medtronic Sofamor Danek). The Ti cages were 30 TELAMON (Medtronic Sofamor Danek) and 2 CFC (DePuy Synthes).
All patients were braced using a Damen corset or semi-hard brace for three months after surgery.
Assessment of bony union
The status of bony union was evaluated by computed tomography (CT) with coronal and sagittal reconstruction according to the classification described by Ito et al. [2010]. In grade 1, complete fusion is achieved with bone bridge formation between the upper and lower vertebral bodies. In grade 2, the bone bridge is not formed, with no translucency observed around the cages, and with thick fusion mass formation. In grade 3, fusion is not achieved, with translucency seen around the cages. In grade 4, the cage sinks into the vertebral body or there is bone resorption around cages indicating pseudo-arthrosis (19). More than 3° of angular motion on flexion-extension in the radiograph was considered to indicate nonunion (15). Therefore, we defined bone union as (I) grade 1 or 2 on both sagittal and coronal CT-multi planer reconstruction image and (II) less than 3° of motion on flexion-extension in the radiograph.
Risk factors
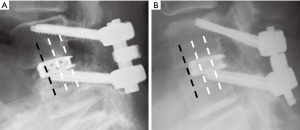
We examined the following factors for their relation to the occurrence of bone union: (I) age; (II) gender; (III) bone mineral density (BMD) (at femoral neck); (IV) cage materials [polyether-ether-ketone (PEEK) or titanium (Ti)]; (V) vertebral-slip (neutral; mm); (VI) translational motion (flexion/extension; mm); (VII) angular motion (flexion/extension; degrees); (VIII) screw depth in the vertebral body (% depth) (Figure 1); (IX) interval of bilateral screw heads (mm), and; (X) cage position (Figure 2). Vertebral slip was measured as the displacement of the vertebral body on the adjacent level below in the anterior or posterior direction in the neutral radiograph. Translational motion was defined as an anterior to posterior shift of the vertebral body between flexion and extension radiographs. Angular motion was defined as the angle of difference between each vertebral body in flexion and extension. The percent depth (% depth) was defined as the percentage of the screw length showed into vertebra in the axial CT (Figure 1). Cage position was categorized into two groups, depending on whether or not the anterior margin of the cage was at the most anterior quarter of the vertebra or not (Figure 2).

Statistical analysis
Continuous variables were analyzed by a paired t-test. Categorical variables were analyzed by chi-square tests. To analyze independent risk factors of bone union, a multivariate logistic regression analysis was conducted by variables with P<0.20 in univariate analysis. All statistical analyses were performed by JMP version 11 (SAS, Cary, NC) and P<0.05 was considered statistically significant.
Results
At two years post-operation, the bone union rate was 88.4% (61/69) of cases.
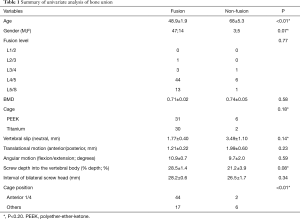
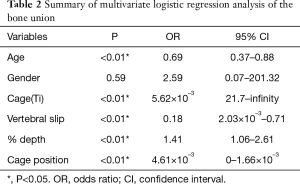
Univariate analysis revealed that variables with P<0.20 were age (P<0.01), gender (P=0.07), cage material (P=0.18), vertebral slip (neutral) (P=0.14), % depth (P=0.086), and cage position (P<0.01) (Table 1). Multiple logistic regression analyses were conducted with these variables and the following independent factors for bone union were identified: age [odds ratio (OR) =0.69, 95% confidence interval (CI), 0.37–0.88, P<0.01], Ti cage (OR =5.62×10–3, 95% CI, 21.7–infinity, P<0.01), vertebral slip (neutral) (OR =0.18, 95% CI, 2.03×10–3–0.71, P<0.01), % depth (OR =1.41, 95% CI, 1.06–2.61, P<0.01), cage position (OR =4.61×10–3, 95% CI, 0–1.66×10–3, P<0.01) (Table 2).
Full table
Full table
In terms of the surgical technique, % depth and cage position were important factors for bone union after CBT-PLIF.
Discussion
In this study, we investigated bone union after CBT-PLIF using radiological assessment and evaluated factors that may influence bone union. Significant factors for successful bone union after CBT-PLIF were (I) young age, (II) small vertebral slip, (III) Ti cage material, (IV) deeper screw insertion into the vertebral body, and (V) an anterior cage position.
Previous studies reported a bone union rate of 70–82.8% using the conventional open technique (15,20), 87.5% using anterior lumbar interbody fusion with percutaneous pedicle screw technique (ALIF with PPS) (21), and 88.4% using the CBT technique (16). In this study, the bone union rate at two years after CBT-PLIF was 88.4% (61/69) of cases. Although the follow-up period and methods of evaluating bone union differed, this bone union rate was similar to those reported in other studies.
Elderly patients showed poor bone union rates in this study. Generally, the bone strength of elderly patients is low, and there are the risks of failed spinal fusion associated with malnutrition, dementia, low activity, medical comorbidities, and osteoporosis. In particular, osteoporotic bone shows irregular trabeculae and insufficient mineral uptake, which may reduce bone strength and the promotion of bone union (14). However, our results showed that BMD (g/cm2), as measured by dual energy X-ray absorptiometry (DEXA), is not a significant factor affecting bone union. DEXA results are increased by degenerative change in bone, thus BMD measured by DEXA may have limitations in the assessment of bone (22). Recently, the Hounsfield unit (HU) scale has been suggested as useful for bone quality assessment and as a predictor of spinal surgery results in elderly patients (23,24). In future studies, an assessment of HU in regards to bone union after PLIF should be performed.
This study showed that spondylolisthesis had less effect on less bone fusion. Hayashi et al. reported the relationship between the Modic type change and motion characteristics in the lumbar spine. They revealed that translational motion increased with Modic type 2 (25). Zhao et al. reported spinal instability associated with endplate disruption in a cadaveric study (26). Although translational and angulation motions were not significant factors for bone union in our study, we thought that potential instability and endplate fragility of spondylolisthesis might lead to less bone union.
Although some studies reported an association between cage material and bone union, the influence of cage material on bone union is unclear (27,28). In the present study, at two years after single-level CBT-PLIF, the fusion rate of the Ti group was 93.7% and that of the PEEK group was 83.7% (P=0.18). Although PEEK has a Young’s modulus similar to that of cortical bone (29), the surface profile of the PEEK cage has smaller teeth than that of the Ti cage (30). This suggests that the primary stability of the PEEK cage is inferior to that of the Ti cage because of insufficient anchoring to the vertebral endplate. Insufficient primary stability leads to micromotion between the cage and vertebral endplate, which may overcome the biomechanical superiority of the PEEK cage. In an in vivo study, more mature osteoblasts were observed on Ti than on PEEK (31). These results suggest that the biocompatibility of PEEK is inferior to that of Ti, and that the Ti cage may provide a more osteogenic surface for interbody fusion. Together, these results suggest that the Ti cage may be superior to the PEEK cage with regard to the fusion rate. In particular, because the anterior support of the vertebral body is the insufficient in CBT-PLIF compared to conventional PLIF, this characteristic and the insufficient primary stability of the PEEK cage might result in a poor bone union rate in addition to the inferior biocompatibility of PEEK.
Mckinley et al. reported that a short screw length into the vertebral body increases the intrapedicular moment (32). This suggests that insufficient screw depth leads to unsuitable load sharing for PLIF constructs. Using the original CBT technique, the screw tip is located locates in the posterior of the vertebral body (1). Therefore, support of the anterior of the vertebral body may be insufficient. In this study, deeper screw insertion into the vertebra contributes to bone union. Sufficient screw length can achieve effective load transmission, and potentially increase the bone union rate. However, deeper CBT screw insertion results in the screw trajectory approaching close to the pedicle axis. Matsukawa et al. evaluated screw insertion torque and screw position in screw fixation by the CBT technique (4). They found that maximization of screw contact with the lamina, which is rich in cortical bone, was important for rigid fixation. They described the optimum trajectory as (I) a starting point in the lower spot of the pars interarticularis; (II) the screw passing through the inferior border of the pedicle, and (III) the screw tip reaching from one-third to one-half of the posterior vertebral body. Therefore, we presumed that acquiring screw depth with an optimal cortical trajectory was ideal for fixation and a satisfactory bone union rate. However, fluoroscopic imaging or a navigation system is required to achieve an optimal cortical trajectory because it is difficult to insert the screw without guidance as performed using conventional techniques.
We revealed that an anterior cage position tended to result in a high bone union rate. Because the position of the screw tip by CBT technique is posterior compared to that of conventional pedicle screw techniques, anterior support of the vertebral body becomes insufficient. Thus, we thought that an anterior cage placement leaded to adequate load sharing for the vertebral body and increased the bone union rate. Our results suggest that cage position is a significant factor in bone union rates, and that an anterior cage position improves the bone union rate.
Overall, our present results suggested that both deeper screw insertion in the vertebral body and anterior cage placement both improved mechanical load transmission and achieved a high bone union rate in CBT-PLIF.
Our study has several limitations. First, this was a retrospective study. A randomized control study should be performed to compare the outcomes using Ti or PEEK cages with an adequate CBT technique. Second, the patients’ clinical outcomes were not assessed. Clinical outcomes are necessary for long-term follow-up. Further research is needed to confirm the relationship between clinical outcomes and bone union after CBT-PLIF. Third, differences in cage shape were not assessed. Cage angle and teeth shape may influence primary stability. It is necessary to investigate the effects of cage shape in order to understand differences in radiographic and clinical outcomes with CBT-PLIF using a single material cage. Fourth, patient age, clinical pathology, and fusion levels vary between subjects. Limiting the subject to a specific age range and inclusive clinical pathologies should lead to a more reliable investigation of factors associated with bone union.
Conclusions
Our results suggest that the following important technical points should be considered when using CBT-PLIF: (I) the screw should be inserted into the vertebra more anteriorly with sufficient cortical bone contact and (II) the cage position should be as anterior as possible. Using these methods, we are able to obtain adequate load sharing for the vertebral body, which may lead to an increased rate of successful bone union. Further, using a Ti cage may lead to a higher bone union rate than a PEEK cage.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-608
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-608
Peer Review File: Available at http://dx.doi.org/10.21037/jss-20-608
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-608). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was ethically approved by the Research Ethics Committee of Murayama Medical Center (receipt ID: 12-10) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Santoni BG, Hynes RA, McGilvary KC, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J 2009;9:366-73. [Crossref] [PubMed]
- Matsukawa K, Yato Y, Kato T, et al. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976) 2014;39:E240-5. [Crossref] [PubMed]
- Matsukawa K, Kato T, Yato Y, et al. Incidence and risk factors of adjacent cranial facet joint violation following pedicle screw insertion using cortical bone trajectory. Spine (Phila Pa 1976) 2016;41:E851-6. [Crossref] [PubMed]
- Matsukawa K, Taguchi E, Yato Y, et al. Evaluation of the fixation strength of pedicle screws using cortical bone trajectory: What is the ideal trajectory for optimal fixation? Spine (Phila Pa 1976) 2015;40:E873-8. [Crossref] [PubMed]
- Djurasovic M, Glassman SD, Dimar JR 2nd, et al. Does fusion status correlate with patient outcome in lumbar spinal fusion? Spine (Phila Pa 1976) 2011;36:404-9. [Crossref] [PubMed]
- Lamberg TS, Remes VM, Helenius IJ, et al. Long-term clinical, functional and radiological outcome 21 years after posterior or posterolateral fusion in childhood and adolescence isthmic spondylolisthesis. Eur Spine J 2005;14:639-44. [Crossref] [PubMed]
- Okuda S, Oda T, Miyauchi A, et al. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am 2006;88:2714-20. [Crossref] [PubMed]
- Kimura H, Shikata J, Odate S, et al. Risk factors for Cage Retropulsion after posterior lumbar interbody fusion: analysis of 1070 cases. Spine (Phila Pa 1976) 2012;37:1164-9. [Crossref] [PubMed]
- Makino T, Kaito T, Fujiwara H, et al. Risk factors for poor patient-reported quality of life outcomes after posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2017;42:1502-10. [Crossref] [PubMed]
- Carpenter CT, Dietz JW, Leung KY, et al. Repair of pseudarthrosis of the lumbar spine: a functional outcome study. J Bone Joint Surg AM 1996;78:712-20. [Crossref] [PubMed]
- Inaoka M, Tada K, Yonenobu K. Problems of posterior lumbar interbody fusion (PLIF) for the rheumatoid spondylitis of the lumbar spine. Arch Orthop Trauma Surg 2002;122:73-9. [Crossref] [PubMed]
- Sawin PD, Dickman CA, Crawford NR, et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg 2001;94:76-81. [PubMed]
- Hermann PC, Webler M, Bornemann R, et al. Influence of smoking on spinal fusion after spondylodesis surgery: A comparative clinical study. Technol Health Care 2016;24:737-44. [Crossref] [PubMed]
- Andersen T, Christensen FB, Langdahl BL, et al. Fusion mass bone quality after uninstrumented spinal fusion in older patients. Eur Spine J 2010;19:2200-8. [Crossref] [PubMed]
- Fujibayashi S, Takemoto M, Izeki M, et al. Does the formation of vertebral endplate cysts predict nonunion after lumbar interbody fusion? Spine (Phila Pa 1976) 2012;37:E1197-202. [Crossref] [PubMed]
- Sakaura H, Miwa T, Yamashita T, et al. Posterior lumbar interbody fusion with cortical bone trajectory fixation versus posterior lumbar interbody fusion using traditional pedicle screw fixation for degenerative lumbar spondylolisthesis: a comparative study. J Neurosurg Spine 2016;25:591-5. [Crossref] [PubMed]
- Kaito T, Fujiwara H, Makino T, et al. Strength and limitations of pedicle screw fixation with cortical bone trajectory. Orthopaedic Surgery and Traumatology 2014;57:1583-9.
- Matsukawa K, Yato Y, Nemoto O, et al. Morphometric measurement of cortical bone trajectory for lumbar pedicle screw insertion using computed tomography. J Spinal Disord Tech 2013;26:E248-53. [Crossref] [PubMed]
- Ito Z, Matsuyama Y, Sakai Y, et al. Bone union rate with autologous iliac versus local bone graft in posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2010;35:E1101-5. [Crossref] [PubMed]
- Kanemura T, Ishikawa Y, Matsumoto A, et al. The maturation of grafted bone after posterior lumbar interbody fusion with an interbody carbon cage. a prospective five-year study. J Bone Joint Surg Br 2011;93:1638-45. [Crossref] [PubMed]
- Lee DY, Lee SH, Maeng DH. Two-level anterior lumbar interbody fusion with percutaneous pedicle screw fixation: a minimum 3-year follow-up study. Neurol Med Chir (Tokyo) 2010;50:645-50. [Crossref] [PubMed]
- Bühler DW, Berlemann U, Oxland TR, et al. Moments and forces during pedicle screw insertion. In vitro and in vivo measurements. Spine (Phila Pa 1976) 1998;23:1220-7; discussion 1228. [Crossref] [PubMed]
- Matsukawa K, Abe Y, Yanai Y, et al. Regional Hounsfield unit measurement of screw trajectory for predicting pedicle screw fixation using cortical bone trajectory: a retrospective cohort study. Acta Neurochir (Wien) 2018;160:405-11. [Crossref] [PubMed]
- Schreiber JJ, Hughes AP, Taher F, et al. An association can be found between Hounsfield units and success of lumbar spine fusion. HSS J 2014;10:25-9. [Crossref] [PubMed]
- Hayashi T, Daubs MD, Suzuki A, et al. Motion characteristics and related factors of Modic changes in the lumbar spine. J Neurosurg Spine 2015;22:511-7. [Crossref] [PubMed]
- Zhao F, Pollintine P, Hole BD, et al. Discogenic origins of spinal instability. Spine (Phila Pa 1976) 2005;30:2621-30. [Crossref] [PubMed]
- Nemoto O, Asazuma T, Yato Y, et al. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cage or titanium cage with transpedicular instrumentation. Eur spine J 2014;23:2150-5. [Crossref] [PubMed]
- Tanida S, Fujibayshi S, Otsuki B, et al. Vertebral endplate cyst as a predictor of nonunion after lumbar interbody fusion. Spine (Phila Pa 1976) 2016;41:E1216-22. [Crossref] [PubMed]
- Vadapalli S, Sairyo K, Goel VK, et al. Biomechanical rationale for using polyetheretherketone (PEEK) spacers for lumbar interbody fusion-A finite element study. Spine (Phila Pa 1976) 2006;31:E992-8. [Crossref] [PubMed]
- Schimmel JJ, Poeschmann MS, Horsting PP, et al. PEEK cages in lumbar interbody fusion: mid-term clinical outcome and radiological fusion. Clin Spine Surg 2016;29:E252-8. [Crossref] [PubMed]
- Navarrete RO, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J 2012;12:265-72. [Crossref] [PubMed]
- McKinley TO, McLain RF, Yerby SA, et al. Characteristics of pedicle screw loading. Effect of surgical technique on intravertebral and intrapedicular bending moments. Spine (Phila Pa 1976) 1999;24:18-24, discussion 25. [Crossref] [PubMed]


