
The influence of sagittal spinopelvic alignment on patient discharge disposition following minimally invasive lumbar interbody fusion
Introduction
Because of the Center for Medicare and Medicaid Services’ initiative towards Alternative Payment Models, surgical specialties may now receive a fixed payment for a 90-day episode-of-care (1), centered around a given operation. Orthopedic surgeons quickly identified that “post-discharge care accounted for more than one-third of total episode payments and varied substantially across patients and procedures” for total joint arthroplasty (2). Kahn et al. followed suit with a cross-sectional cohort of 17,436 spine surgery episodes among 50 hospitals. The authors concluded that 32.5% of overall variation between the highest and lowest spending quintile hospitals was attributed to post-discharge payments (3). The surgical literature has subsequently shifted focus to predisposing factors that lead to rehabilitation facility admission following lumbar fusion surgery.
Medical prognostic factors have been elucidated as the strongest predictors of discharge status in patients undergoing lumbar spine surgery (4-8). While researchers have attended to age, increased body mass index, increased length of stay, increased operative time, increased disability scores, and increased American Society of Anesthesiologists (ASA) class in prediction models, surgical technique and operative planning may also play a pivotal role post-acute care disposition.
Because the correction of spinopelvic alignment in adult deformity surgery has been associated with improved functional status, health-related quality of life outcomes, and patient satisfaction (9-14), we hypothesized that these parameters may also affect postoperative discharge disposition. According to Glassman et al.’s assessment of multiple radiographic parameters on health status measures in a large population of deformity patients, positive sagittal balance prevailed as the greatest prognosticator of health status measures (15). As such, sagittal balance may also affect discharge disposition; however, this potential relationship has been poorly established in the literature. The objective of this study was to investigate the changes to spinopelvic sagittal alignment following minimally invasive (MIS) lumbar interbody fusion and the influence of such changes on postoperative discharge disposition. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-596).
Methods
Study design, setting, and participants
The Michigan Spine Surgery Improvement Collaborative (MSSIC) is a statewide, prospective, multicenter quality improvement initiative (16-18). MSSIC includes a comprehensive longitudinal registry aimed at enhancing clinical quality, identifying, and decreasing complications, and improving overall patient outcomes in spine surgery (16-18). The use of MSSIC’s database of more than 40,000 patients from 26 participating hospitals to complete retrospective cohort studies has been well established (16,17). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Henry Ford Hospital (IRB No. 10632) and individual consent for this retrospective analysis was waived.
Variables and data sources/measurement
Following Institutional Review Board approval (No. 10582), MSSIC was queried for all patients who underwent transforaminal (TLIF) or lateral lumbar interbody (LLIF) fusion procedures for degenerative spine disease between 2016 and 2017. Because physicians and institutions differ in discharge practices, patients were limited to a single surgeon’s experience to maintain internal validity. Cases were manually abstracted from the daily operative schedule at our institution. Cases were limited to 1- or 2-level operations.
Preoperative demographic data, intraoperative parameters, and 90-day postoperative complications by a single-surgeon were abstracted. Perioperative demographic data included the ASA classification that serves as a surrogate marker of comorbidity burden. However, the misclassification of ASA risks by anesthesia personnel represents an inherent weakness of the five-category physical status system. Alternatively, the burden of preoperative morbidity followed an algorithm of matching 16 variables in the National Surgical Quality Improvement Program (NSQIP) database to 11 corresponding items in the Canadian Study of Health and Aging Frailty Index, which in turn was used to calculate a modified Frailty Index (mFI) (19). Developed by Tsiouris et al. (20), this prevalidated risk assessment assigns one point to each of the following variables: non-independent functional status, diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, myocardial infarction, coronary artery disease (coronary intervention, cardiac surgery, or angina), hypertension requiring medication, peripheral vascular disease, impaired sensorium, transient ischemic attack or stroke without neurological deficit, and stroke with neurological deficit. The sum of points is divided by 11 to obtain a mFI score (0–1.0), wherein higher scores presume a greater comorbidity burden.
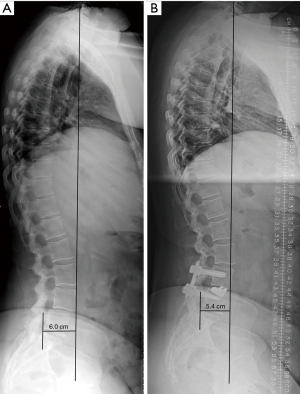
Radiographic evaluation of all patients was performed by neurosurgery residents blinded to the subject’s clinical information. Lateral and anteroposterior 36-inch upright standing radiographs were obtained preoperatively and within three months following surgery. Several spinopelvic sagittal alignment parameters were measured, including: sagittal vertical axis (SVA), lumbar lordosis (LL), pelvic tilt (PT), pelvic incidence (PI), and pelvic incidence-lumbar lordosis (PI-LL) mismatch. SVA was measured as the horizontal distance from the C7 plumb line to the posterior superior aspect of the S1 endplate (Figure 1). LL was determined using the Cobb angle between the superior endplate of L1 and the superior endplate of S1. Pelvic tilt refers to the angle subtended by a vertical reference line at the femoral head and the midpoint of the rostral S1 vertebral body to the femoral head. The pelvic tilt complements the pelvic incidence, which was measured as the angle subtended by a line from the femoral head to the midpoint of the rostral S1 sacrum and a line perpendicular to the rostral S1 sacrum. PI-LL mismatch was the difference between the measurements of PI and LL.
Categorical variables
The primary outcome measure reflected the likelihood of discharge to a rehabilitation facility. Patients included in the study were placed into one of two cohorts: discharge to home or discharge to rehabilitation facility. Patients placed into the discharge home cohort were discharged directly to their home following surgery. Patients placed into the discharge to rehabilitation facility cohort were discharged to a skilled nursing facility, subacute rehabilitation facility, extended rehabilitation facility or nursing home following surgery.
Statistical methods
The rehabilitation cohort was compared to the home cohort, reporting means ± standard deviations (SD) or frequencies/percentages. Means of continuous variables were first calculated with a variance ratio test. Continuous data that followed a Gaussian distribution were compared using a Student’s t-test; non-normally distributed numbers were calculated with Welch’s test. Binary outcomes were compared with a chi-squared (χ2) test, and median/ordinal outcomes with a Mann-Whitney U test (Wilcoxon Rank-Sum Test). According to the methods described in a previous publication (21-23), the logistic regression model was fitted to the data to estimate the effect of discharge to rehabilitation. Associations between prognostic factors and primary outcomes were calculated with adjusted odds ratio (ORadj) in the multivariable logistical regression. Because SVA was the only spinal alignment parameter that statistically significantly differed between the discharge to home cohort versus the discharge to rehab cohort, SVA was selected as the radiographic measurement for inclusion in the multivariable regression analysis. All odds ratios were described with 95% confidence intervals (CI). Statistical significance was set at P≤0.05. Lastly, a margins plot illustrated the effect of spinal alignment on the probability of discharge to rehab (95% CI) when controlling for other variables in the logistical regression.
Sensitivity analysis
As detailed in a prior publication (24), after an initial univariable logistical regression, predictors of discharge disposition were calculated with multivariable logistical regression with a forward stepwise modeling, reporting ORadj. Each regression model tested was analyzed with a sensitivity analysis based on three statistical approaches: (I) Akaike’s Information Criterion (AIC); (II) model discrimination: C-statistic corresponding to the area under the receiver-operating characteristic (ROC) curve; and (III) model calibration: P value of the Hosmer-Lemeshow Goodness-of-Fit test. The AIC provides a tradeoff between the log-likelihood function and the number of covariables, such that smaller AIC values suggest a more robust model fit (25). The C-statistic reflects model discrimination. By approximating the ROC curve, a C-statistic measures how well the regression model can discriminate among different observations and the primary outcome measure: disposition (26). According to the Hosmer and Lemeshow principles on the C-statistic, discrimination can be defined as acceptable (0.7–0.8), excellent (0.8–0.9), or outstanding (≥0.9) (27). Lastly, the Hosmer-Lemeshow approach tests for goodness-of-fit of the model, or model calibration. This statistic resembles a chi-square test modified with the degrees of freedom (28). A Hosmer-Lemeshow Goodness-of-Fit test approaching a P value of zero means a poor fit of the data.
Results
Participants and descriptive data
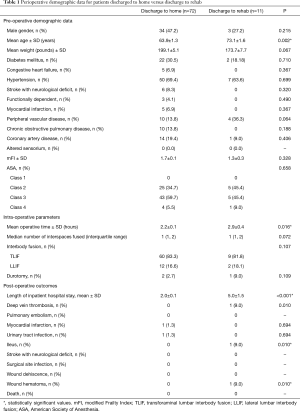
Of the 83 patients in the study population, 11 (13.2%) were discharged to rehab (Table 1). The mean age of 73.1±1.6 years in the discharge-to-rehab cohort was statistically significantly older than the mean age of 63.9±1.3 years in the discharge-to-home cohort (Welch’s t-test, P=0.002). All other preoperative demographic data—male gender (P=0.215) and weight (P=0.067)—did not differ. Statistical significance was not reached with all measured comorbidities, including diabetes mellitus (P=0.710), congestive heart failure (P=0.367), hypertension (P=0.699), stroke with neurological deficit (P=0.320), functional dependency (P=0.490), myocardial infarction (P=0.367), peripheral vascular disease (P=0.064), chronic obstructive pulmonary disease (P=0.188), coronary artery disease (P=0.406), and altered sensorium (0%). As such, the discharge-to-home versus discharge-to-rehab cohorts did not differ with surrogate markers of comorbidity burden: mFI (P=0.328) and ASA Classification (P=0.658).
Full table
In intraoperative parameters, surgical time among patients discharged to rehab averaged to 2.9±0.4 hours, which was statistically significantly longer than the 2.2±0.1 hours among patients discharged to home (P=0.016). All remaining intraoperative parameters did not differ. In postoperative outcomes, mean length of inpatient hospital stay was as expected more than double among patients discharged to rehab (5.0±1.5 days) versus discharged to home (2.0±0.1 days) (P<0.001). The discharge-to-rehab versus discharge-to-home division saw one deep vein thrombosis (9% vs. 0%, P=0.010), one ileus (9% vs. 0%, P=0.010), and one wound hematoma (9% vs. 0%, P=0.010). No other complications statistically differed.
Outcome data
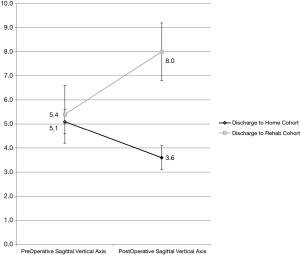
In spinal alignment parameters, the preoperative SVA of 5.4 cm in the discharge-to-rehabilitation cohort did not statistically significantly differ from 5.1 cm in the discharge-to-home cohort (Table 2, Figure 2). As compared to preoperative measurements, the postoperative SVA increased to 8.0 cm in the discharge-to-rehab division versus decrease to 3.6 cm in the discharge-to-home division (P<0.001).

Full table
Main results
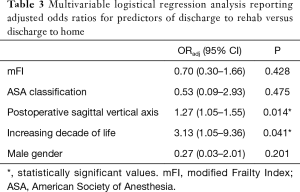
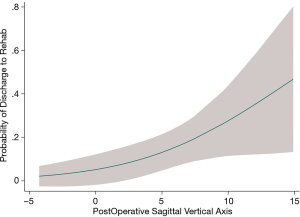
Following a multivariable logistical regression controlling for mFI and male gender, the odds of discharge to rehab increases by 25% for every 1 cm increase in postoperative sagittal balance (ORadj =1.27, P=0.014) (Table 3). The strongest predictor of discharge to rehab was increasing decade of life (ORadj =3.13, P=0.041). A margins plot demonstrates increasing probability of discharge to rehab with increasing postoperative SVA, when controlling for mFI, ASA classification, increasing decade of life, and male gender (Figure 3).
Full table
Sensitivity analysis
Multiple logistical regression modeling was based on three parameters: (I) AIC; (II) model discrimination via the C-statistic; and (III) model calibration via the Hosmer-Lemeshow Goodness-of-Fit test (24). Of the three models enumerated in Table 4, a multiple logistical regression controlling for mFI, ASA Classification, postoperative SVA, increasing decade of life, and male gender (model 1) returned the lowest AIC of 54.45, suggesting the best model fit. With respect to model discrimination, the ROC curve for model 1 yielded a C-statistic of 0.87, which upholds excellent discrimination. Lastly, with respect to model calibration, the p-value of the Hosmer-Lemeshow Goodness-of-Fit test was not statistically significant (P=0.938). Adding covariates in multiple logistical regression modeling via a forward stepwise approach increased the AIC and/or decreased the C-statistic; therefore, a multiple regression model with the five covariates—mFI, ASA Classification, postoperative SVA, increasing decade of life, and male gender—was selected for the regression analysis in Table 3.

Full table
Discussion
Key results
In the current study of patients undergoing LLIF or TLIF operations, preoperative SVA was equivalent among patient discharged to home versus rehab. Interestingly, the former group exhibited greater correction on postoperative SVA. In the regression analysis, every 1-cm improvement in postoperative sagittal balance increased the odds of discharge to home by 27%.
Interpretation
Only a paucity of studies has investigated the effect of spinal alignment parameters on the discharge disposition of patients undergoing spinal fusion surgery. Our study indicates that patients with a higher sagittal balance following MIS lumbar interbody fusion for degenerative spine disease equated to higher rates of discharge to a rehabilitation facility versus home. Following a multivariable logistical regression analysis, the odds of discharge to rehabilitation facility increase by 27% for every 1 cm increase in postoperative sagittal alignment (Table 3). Because discharge disposition serves as a surrogate for favorable postoperative outcomes, our findings may profoundly alter patient outcomes (8). Multiple studies have found that patients discharged to a rehabilitation facility following lumbar fusion procedures have higher mortality rates, higher risks of minor and major postoperative complications (i.e., wound complications, infectious complications, pulmonary embolism, and myocardial infarction, etc.), higher readmission rates, and higher risks of returning to the operating room (5,29-32).
This study is unique in that, although preoperative SVA was equivalent between the home cohort and the rehabilitation facility cohort, patients discharged to a rehabilitation facility incurred a postoperative SVA significantly higher (8.0±1.3 cm) than patients discharged home (3.6±0.4 cm). This novel finding corroborates the established relationship between sagittal misalignment and poor outcomes seen in patients with adult spinal deformity. Glassman et al., by evaluating 352 patients with adult spinal deformity, first reported that positive sagittal alignment (mean SVA 57.7±51.2 mm) was linearly associated with pain and poor self-reported health-related quality of life measures (15). Subsequently, a wealth of studies reliably established an association of sagittal misalignment with postoperative pain and disability (9-14,33). These unfavorable outcomes undoubtedly hinder a working relationship between the patient and the physical therapist. In a prospective longitudinal study of patients undergoing spine surgery, Skolasky et al. investigated a postoperative patient’s propensity to engage in adaptive health behaviors, or “patient activation,” with the physical therapist (34). The Hopkins Rehabilitation Engagement Rating Scale—an objective and quantitative surrogate of patient activation—was most strongly correlated with postoperative patient-reported outcomes, specifically optimism and self-efficacy. The lack of patient activation then leans towards a discharge to a rehabilitation facility.
Our research on the unique correlation between sagittal balance and discharge disposition underscores how the surgical management of patients with degenerative spinal disease has adopted the principles of deformity surgery. Multiple publications demonstrate that at least 40% of patients suffering from lumbar spondylolisthesis, spondylosis, degenerative disc disease, and canal stenosis exhibit sagittal misalignment (SVA ≥5 cm) (35-39). Jagannathan et al. first demonstrated that an improvement in sagittal alignment can be achieved following short-segment TLIF in 80 patients with lumbar degenerative spine disease (38). Subsequently, Cheng et al. retrospectively examined 92 patients who underwent single-level TLIF to treat symptomatic lumbar degenerative spine disease (40). Patients were divided into three groups based on preoperative symptoms (1: low back pain, 2: radiculopathy, 3: neurogenic claudication) and robust preoperative and postoperative radiographic measurements were taken (40). On long-term follow-up, clinical parameters measured by visual analog scales and the Oswestry Disability Index significantly improved following TLIF in all three groups. Sagittal alignment only significantly improved in the radiculopathy (ΔSVA −7.0±9.2 mm) and neurogenic claudication groups (ΔSVA −21.5±17.6 mm), with both groups achieving an SVA <50 mm (40). Building from these previous reports, our study found that 87% of MIS interbody fusion procedures resulted in significant improvement in SVA and subsequent discharge home rather than to a rehabilitation facility. Considering the established relationship between sagittal alignment and postoperative functional status, health-related quality of life outcomes, and patient satisfaction, our study is the first to also associate improved postoperative sagittal alignment with favorable discharge disposition.
Although sagittal alignment was independently associated with favorable discharge disposition in our study, prior studies have found patient age as one of the strongest independent predictors of postoperative discharge to a rehabilitation facility after lumbar fusion surgery (7,8,29,30). This finding was echoed in our statistical analysis, wherein the mean age of our patients discharged to a rehabilitation facility (73.1±1.6 years) was significantly older than the patients discharged home (63.9±1.3 years) (Table 1). Following a multivariable logistic regression, age yields the strongest predictor of discharge to a rehabilitation facility with every decade of life increasing the odds of rehab discharge by three times (Table 3).
In conclusion, the findings of our study, coupled with the recognized negative consequences of admission to a rehabilitation facility, can serve to guide and inform preoperative patient assessments, surgical decision making, and postoperative disposition planning for patients undergoing MIS lumbar interbody fusion for degenerative spine disease.
Limitations and generalizability
One limitation of this study is the statistically significant difference in operative time between the discharge to home cohort versus discharge to a rehabilitation facility cohort. While this difference may reflect a discrepancy in case complexity between the two groups, qualifying case difficulty was beyond the scope of this study. Calculation of radiographic parameters was limited to only one reading by a neurosurgery resident. Neither intraclass nor interclass correlations, or any other reliability assessments, were calculated. In terms of patient symptomatology, chief complaint—such as radiculopathy, neurogenic claudication, pure axial back pain—was not collected in the current study. Because these presenting symptoms have been included in the regression analyses of prior studies (40), the multivariable analysis presented in Table 3 is subject to an incomplete control of potentially confounding variables.
Our retrospective study design has inherent limitations. As with all retrospective studies, our results are susceptible to an information bias, which was mitigated by standardizing data collection and outcome measures. Future prospective studies ascertaining the effect of spinal alignment parameters and discharge disposition may be required to validate our findings.
Conclusions
Among one of the first reported in the literature, this unique study speaks to the growing impact of sagittal balance on patient outcomes. Of the 83 patients undergoing minimally-invasive lumbar interbody fusion, patients discharged to rehab had a statistically significantly higher SVA as compared to patients discharged to home. Following a multivariable logistical regression, the odds of discharge to rehabilitation increase with a higher postoperative sagittal balance. Although age represented the strongest predictor of discharge disposition, preoperative surgical planning that accounts for spinopelvic alignment will improve the odds of discharge to home postoperatively.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-596
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-596
Peer Review File: Available at http://dx.doi.org/10.21037/jss-20-596
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-596). VC has consulting agreements with DepuySynthes and Globus, and receives some research support from Medtronic. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Henry Ford Hospital (IRB No. 10632) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cutler DM, Ghosh K. The Potential for Cost Savings through Bundled Episode Payments. N Engl J Med 2012;366:1075-7. [Crossref] [PubMed]
- Bozic KJ, Ward L, Vail TP, et al. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res 2014;472:188-93. [Crossref] [PubMed]
- Kahn EN, Ellimoottil C, Dupree JM, et al. Variation in payments for spine surgery episodes of care: implications for episode-based bundled payment. J Neurosurg Spine 2018;29:214-9. [Crossref] [PubMed]
- Aldebeyan S, Aoude A, Fortin M, et al. Predictors of Discharge Destination After Lumbar Spine Fusion Surgery. Spine (Phila Pa 1976) 2016;41:1535-41. [Crossref] [PubMed]
- Arrighi-Allisan AE, Neifert SN, Gal JS, et al. Discharge Destination as a Predictor of Postoperative Outcomes and Readmission Following Posterior Lumbar Fusion. World Neurosurg 2019;122:e139-46. [Crossref] [PubMed]
- McGirt MJ, Parker SL, Chotai S, et al. Predictors of extended length of stay, discharge to inpatient rehab, and hospital readmission following elective lumbar spine surgery: introduction of the Carolina-Semmes Grading Scale. J Neurosurg Spine 2017;27:382-90. [Crossref] [PubMed]
- Morcos MW, Jiang F, McIntosh G, et al. Predictive Factors for Discharge Destination Following Posterior Lumbar Spinal Fusion: A Canadian Spine Outcome and Research Network (CSORN) Study. Global Spine J 2019;9:403-8. [Crossref] [PubMed]
- Murphy ME, Maloney PR, McCutcheon BA, et al. Predictors of Discharge to a Nonhome Facility in Patients Undergoing Lumbar Decompression Without Fusion for Degenerative Spine Disease. Neurosurgery 2017;81:638-49. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Glassman SD, et al. Clinical and radiographic parameters that distinguish between the best and worst outcomes of scoliosis surgery for adults. Eur Spine J 2013;22:402-10. [Crossref] [PubMed]
- Scheer JK, Lafage R, Schwab FJ, et al. Under Correction of Sagittal Deformities Based on Age-adjusted Alignment Thresholds Leads to Worse Health-related Quality of Life Whereas Over Correction Provides No Additional Benefit. Spine (Phila Pa 1976) 2018;43:388-93. [Crossref] [PubMed]
- Smith JS, Lafage V, Shaffrey CI, et al. Outcomes of Operative and Nonoperative Treatment for Adult Spinal Deformity: A Prospective, Multicenter, Propensity-Matched Cohort Assessment With Minimum 2-Year Follow-up. Neurosurgery 2016;78:851-61. [Crossref] [PubMed]
- Hayashi K, Boissiere L, Guevara-Villazon F, et al. Factors influencing patient satisfaction after adult scoliosis and spinal deformity surgery. J Neurosurg Spine 2019;31:408-17. [Crossref] [PubMed]
- Passias PG, Bortz CA, Lafage V, et al. Durability of Satisfactory Functional Outcomes Following Surgical Adult Spinal Deformity Correction: A 3-Year Survivorship Analysis. Oper Neurosurg (Hagerstown) 2020;18:118-25. [PubMed]
- Ames CP, Smith JS, Scheer JK, et al. Impact of spinopelvic alignment on decision making in deformity surgery in adults: A review. J Neurosurg Spine 2012;16:547-64. [Crossref] [PubMed]
- Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024-9. [Crossref] [PubMed]
- Park P, Nerenz DR, Aleem IS, et al. Risk Factors Associated With 90-Day Readmissions After Degenerative Lumbar Fusion: An Examination of the Michigan Spine Surgery Improvement Collaborative (MSSIC) Registry. Neurosurgery 2019;85:402-8. [Crossref] [PubMed]
- Zakaria HM, Bazydlo M, Schultz L, et al. Adverse events and their risk factors 90 days after cervical spine surgery: analysis from the Michigan Spine Surgery Improvement Collaborative. J Neurosurg Spine 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Chang V, Schwalb JM, Nerenz DR, et al. The Michigan Spine Surgery Improvement Collaborative. a statewide Collaborative Quality Initiative. Neurosurg Focus 2015;39:E7. [Crossref] [PubMed]
- Ali R, Schwalb JM, Nerenz DR, et al. Use of the modified frailty index to predict 30-day morbidity and mortality from spine surgery. J Neurosurg Spine 2016;25:537-41. [Crossref] [PubMed]
- Tsiouris A, Hammoud ZT, Velanovich V, et al. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res 2013;183:40-6. [Crossref] [PubMed]
- Bydon M, Macki M, De la Garza-Ramos R, et al. Smoking as an independent predictor of reoperation after lumbar laminectomy: a study of 500 cases. J Neurosurg Spine 2015;22:288-93. [Crossref] [PubMed]
- Macki M, Fakih M, Kandagatla P, et al. The Impact of Different Postgraduate Year Training in Neurosurgery Residency on 30-Day Return to Operating Room: A National Surgical Quality Improvement Program Study. World Neurosurg 2018;114:e70-6. [Crossref] [PubMed]
- Macki M, Basheer A, Lee I, et al. Surgical site infection after transoral versus posterior approach for atlantoaxial fusion: a matched-cohort study. J Neurosurg Spine 2018;28:33-9. [Crossref] [PubMed]
- Macki M, Uzosike A, Kerezoudis P, et al. Duration of indwelling drain following instrumented posterolateral fusion of the lumbar spine does not predict surgical site infection requiring reoperation. J Clin Neurosci 2017;40:44-8. [Crossref] [PubMed]
- Akaike H. A new look at the statistical model identification. Automatic Control IEEE Transactions on 1974;19:716-23.
- Pepe MS. The statistical evaluation of medical tests for classification and prediction. Oxford: Oxford University Press, 2003.
- Hosmer DW Jr, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons, 2004.
- Hosmer DW, Lemesbow S. Goodness of fit tests for the multiple logistic regression model. Communications in Statistics-Theory and Methods 1980;9:1043-69. [Crossref]
- Khormaee S, Samuel AM, Schairer WW, et al. Discharge to inpatient facilities after lumbar fusion surgery is associated with increased postoperative venous thromboembolism and readmissions. Spine J 2019;19:430-6. [Crossref] [PubMed]
- Malik AT, Kim J, Yu E, et al. Discharge to Inpatient Care Facility After Anterior Lumbar Interbody Fusion: Incidence, Predictors, and Postdischarge Outcomes. World Neurosurg 2019;122:e584-90. [Crossref] [PubMed]
- Cook C, Coronado RA, Bettger JP, et al. The association of discharge destination with 30-day rehospitalization rates among older adults receiving lumbar spinal fusion surgery. Musculoskelet Sci Pract 2018;34:77-82. [Crossref] [PubMed]
- Akamnonu C, Cheriyan T, Goldstein JA, et al. Unplanned hospital readmission after surgical treatment of common lumbar pathologies: rates and causes. Spine (Phila Pa 1976) 2015;40:423-8. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Fu KM, et al. Clinical and radiographic evaluation of the adult spinal deformity patient. Neurosurg Clin N Am 2013;24:143-56. [Crossref] [PubMed]
- Skolasky RL, Mackenzie EJ, Wegener ST, et al. Patient activation and adherence to physical therapy in persons undergoing spine surgery. Spine (Phila Pa 1976) 2008;33:E784-91. [Crossref] [PubMed]
- Shin EK, Kim CH, Chung CK, et al. Sagittal imbalance in patients with lumbar spinal stenosis and outcomes after simple decompression surgery. Spine J 2017;17:175-82. [Crossref] [PubMed]
- Hikata T, Watanabe K, Fujita N, et al. Impact of sagittal spinopelvic alignment on clinical outcomes after decompression surgery for lumbar spinal canal stenosis without coronal imbalance. J Neurosurg Spine 2015;23:451-8. [Crossref] [PubMed]
- Radovanovic I, Urquhart JC, Ganapathy V, et al. Influence of postoperative sagittal balance and spinopelvic parameters on the outcome of patients surgically treated for degenerative lumbar spondylolisthesis. J Neurosurg Spine 2017;26:448-53. [Crossref] [PubMed]
- Jagannathan J, Sansur CA, Oskouian RJ Jr, et al. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery 2009;64:955-63; discussion 963-4. [Crossref] [PubMed]
- Le Huec JC, Faundez A, Dominguez D, et al. Evidence showing the relationship between sagittal balance and clinical outcomes in surgical treatment of degenerative spinal diseases: a literature review. Int Orthop 2015;39:87-95. [Crossref] [PubMed]
- Cheng X, Zhang F, Zhang K, et al. Effect of Single-Level Transforaminal Lumbar Interbody Fusion on Segmental and Overall Lumbar Lordosis in Patients with Lumbar Degenerative Disease. World Neurosurg 2018;109:e244-51. [Crossref] [PubMed]


