
Osteoporosis is under recognized and undertreated in adult spinal deformity patients
Introduction
Adult spinal deformity (ASD) can affect as many as half of all individuals over the age of sixty in the United States (1). While many patients have minimal to no symptoms, others have significant pain and dysfunction that can be compounded by concomitant central stenosis and other forms of nerve compression (2,3). The disease burden associated with ASD has been found to be higher than that of chronic conditions such as diabetes, chronic lung disease, congestive heart failure, and arthritis (3). As a result, many patients undergo surgical treatment that often involves a long spinal fusion for deformity correction (4). Despite recent advances in surgical technique and implant design (5,6), complications such as failure of hardware, proximal junctional kyphosis, and infection commonly arise (7,8).
As with any instrumented fusion, ASD surgery can be further complicated by the presence of osteoporosis, as low bone mineral density presents substantial fixation challenges (9). One study found that 9.5% of all osteoporotic patients also suffer from ASD symptoms (10). Other studies have found the incidence of spinal stenosis, fractures, and progressive deformities occur at higher rates among osteoporotic than non-osteoporotic patients (11,12). However, to our knowledge, no prior study has investigated the prevalence of osteoporosis among patients undergoing long spinal fusions for ASD. Prior study has demonstrated osteoporosis treatment improves outcomes of one level lumbar fusion (13). Extrapolating this to ASD surgery, treatment can only be initiated with detection. Therefore, the purpose of this study was to determine the prevalence of osteoporosis and frequency of its treatment in ASD patients undergoing long fusion.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/jss-20-668).
Methods
ASD patients who underwent surgery at either of two major academic medical centers from 2010 through 2019 were eligible for this study. Patients who were at least 40 years of age at the time of surgery were included. Patients with documented evidence of a spinal tumor were excluded. Through the utilization of a research patient data registry (RPDR), ASD patients were identified via any of the following ICD-10 codes: M41.3X (thoracogenic scoliosis), M41.5X (other secondary scoliosis) M41.8X (other forms of scoliosis), and M41.9X (scoliosis, unspecified). Within this group, patients who endured a spinal fusion of at least seven vertebral levels were filtered using the CPT codes 22843 (posterior segmental instrumentation, 7 to 12 vertebral segments) and 22844 (posterior segmental instrumentation, 13 or more vertebral segments).
Demographic data for each patient was obtained, including a history of osteoporosis (identified using ICD 10 codes M80.XX and M81.XX). Patient records were reviewed for treatment of osteoporosis with any of the following medications: teriparatide, abaloparatide, denosumab, raloxifene, or any bisphosphonate. Patients were considered to be treated for osteoporosis if any of the aforementioned medications was taken during for at least the immediate twelve weeks prior to surgery. The prevalence of osteoporosis who underwent a long spinal fusion was calculated. Demographic data was compared between osteoporotic patients who received treatment for osteoporosis and those who did not.
Statistical analysis
When analyzing categorical variables, such as patient sex or race, chi-square tests were utilized to determine P values. For continuous variables, such as patient age, two-tailed t-tests were utilized for purposes of determining statistical significance. Data was tabulated on the basis of osteoporosis status and whether patients were actively receiving treatment for such.
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by approved by the institutional review board at both medical centers (No: 2018P001897) and individual consent for the retrospective analysis was waived.
Results
Among the initial pool of patients, 399 met inclusion criteria. Within this cohort, 131 patients (32.8%) were diagnosed with osteoporosis before their surgical procedure. Table 1 provides summative demographic data among osteoporotic and non-osteoporotic patients. On average, osteoporotic patients were statistically older than non-osteoporotic patients by approximately three years (66.4 vs. 63.5, P=0.002). Osteoporotic patients also exhibited a higher proportion of females relative to non-osteoporotic patients (74.8% vs. 61.9%, P=0.01). Greater than 90% of patients in this study were Caucasian.

Full table
In total, 34.4% of osteoporotic patients were taking at least one anti-osteoporotic medication, while the majority were untreated. Table 2 depicts comparisons of demographical data between osteoporotic patients who were treated for osteoporosis versus those who were not. Age and race between these groups did not differ, though there were more females on medications than males without reaching statistical significance (P=0.07).

Full table
Table 3 provides information relating to the medical treatment of osteoporosis among the study population. Bisphosphonates and teriparatide were the most commonly used medications, each were taken by approximately one-fifth of osteoporotic patients. On the other hand, both abaloparatide and raloxifene were prescribed to only 1.5% of ASD patients with osteoporosis. While 65.6% of patients did not undergo medical treatment of osteoporosis, 9.2% of patients received multiple medications as part of their treatment. Medication regimens are illustrated graphically in Figure 1.
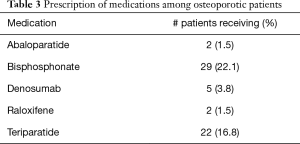
Full table
Discussion
Our data are consistent with well-known demographics of osteoporosis, supporting its generalizability. That is, both older age and the female gender are correlated with the incidence of osteoporosis (15-17). In addition, the current study showed approximately one-third of patients undergoing a long spinal fusion have a diagnosis of osteoporosis. More importantly, two-thirds of these patients were untreated.
The prevalence of osteoporosis in ASD patients reported in this study is higher than previous reports investigating the general population; Wright et al. (1) estimated that 10.3% of Americans above the age 50 are osteoporotic. While this proportion is higher than we would have anticipated, it may indeed underestimate the true incidence of low bone density in this population. Gupta et al. (18) concluded that for accurate measurement of bone mineral density in ASD patients, DEXA scans should be obtained from at least two anatomic locations other than the spine. While the International Society for Clinical Densitometry (ISCD) recommends obtaining DEXA scans from the spine and hip for patients being evaluated for osteoporosis (19), patients with ASD often show falsely elevated bone density in the spine. As a result, osteopenia or osteoporosis is missed in one in six ASD patients (18). This was likely the case in patient cohort as well.
Roh et al. (20) recently showed that there are several reasons patients are not properly evaluated and treated for osteoporosis. Per their analysis, the leading cause for inadequate testing is patients’ fear of finding an osteoporotic fracture. Other factors included patient health literacy, socioeconomic standing, and age, with younger patients less likely to seek testing or start (or continue) treatment for osteoporosis within one year of the diagnosis. These findings further underscore the need for vigilance in osteoporosis detection and treatment in ASD patients.
Beyond detection, previous data have shown that only one fourth of patients with fragility fractures subsequently received osteoporotic treatment consistent with recommended guidelines (21). This is consistent with the multitude of studies that show patients with recently diagnosed fragility fractures in various anatomical locations are most often not treated for osteoporosis, and thus represent the now well-known “missed opportunity (22,23).” Our study further supports this as only 34% of patients with osteoporosis were treated pharmacologically. Furthermore, our data showed that osteoporotic men were less likely to be treated, representing a group in which the opportunity is being disproportionately missed.
Osteoporosis has been shown to increase the rate of complications following spinal surgery (3). Considering that osteoporosis treatment is proven effective at improving bone density (24-27), it is prudent for surgeons to ensure that bone density not only be assessed but that treatment is initiated before surgery. As ASD surgery is already prone to complications, pathways to minimize this risk in an already frail group of patients are important. Further study will be needed to in fact determine the ideal pathway to most positively impact outcomes.
While bisphosphonates can minimize bone loss, anabolic agents can effectively increase bone mass. As such, teriparatide use can lower the risk of vertebral fractures by 65%, and nonvertebral fractures by 35% (28). This agent is much more effective than bisphosphonates in preventing vertebral fractures (29). Despite these clear benefits, our study found that anabolic medications were used in fewer than 20% of ASD patients with osteoporosis. Regardless of the pharmacological treatment choice, patient education is critical to ensuring compliance to achieve adequate bone density optimization preoperatively.
This study has several limitations that should be acknowledged. Our data stems from two large academic centers in the New England region of the United States. Though demographical trends appears generalizable, it may not be widely applicable to other parts of the country or world. In addition, we also did not assess whether or not patients adhered to their medication regimen, as we relied on prescription records. We also were not able to fully assess their progress by virtue of periodic DEXA scans to assess improvement of bone mineral density over time; this data often was not collected. Our study also did not consider use of alternative osteoporotic treatments such as Vitamin D. Lastly, there might have been sound medical reasons that patient were not prescribed anti-osteoporotic medications. Our analysis was not able to detect patients who were osteopenic, and thus could distinguish between these patients and those with normal bone density. If anything, this further underestimated the proportion of patients with compromised bone quality.
Conclusions
The prevalence of osteoporosis in ASD patients undergoing a long spinal fusion is nearly 33%. Just over a third of these patients were treated pharmacologically for osteoporosis, meaning that nearly two thirds were left untreated. Male patients with osteoporosis were less likely to be treated for osteoporosis than women and are identified as a group who are a particularly “missed opportunity” (P=0.07). Given these data, surgeons should have a low threshold to test and treat for osteoporosis in ASD patients undergoing spinal fusions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/jss-20-668
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-668
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-668). Dr. TC reports personal fees from Bio2, Nuvasive, and K2M and institutional fees from NIH, Donoghue, Nuvasive, K2M, and Omega. Dr. CP reports personal fees from Johnson & Johnson. Dr. AH has institutional royalties from Zimmer and Atlas as well as personal consulting fees from Medtronic, Zimmer, and Atlas. Dr. CMB reports personal fees from Wolters Kluwer, Elsevier, and United Healthcare. Dr. SH reports personal fees from Depuy and Nuvasive and institutional fees from Nuvasive, K2M, and Omega. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by approved by the institutional review board at both medical centers (No: 2018P001897) and individual consent for the retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wright NC, Looker AC, Saag KG, et al. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J Bone Miner Res 2014;29:2520-6. [Crossref] [PubMed]
- Kebaish KM, Neubauer PR, Voros GD, et al. Scoliosis in adults aged forty years and older: prevalence and relationship to age, race, and gender. Spine (Phila Pa 1976) 2011;36:731-6. [Crossref] [PubMed]
- Soroceanu A, Burton DC, Oren JH, et al. Medical Complications After Adult Spinal Deformity Surgery: Incidence, Risk Factors, and Clinical Impact. Spine (Phila Pa 1976) 2016;41:1718-23. [Crossref] [PubMed]
- Pellisé F, Vila-Casademunt A, Ferrer M, et al. Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur Spine J 2015;24:3-11. [Crossref] [PubMed]
- Auerbach JD, Lenke LG, Bridwell KH, et al. Major complications and comparison between 3-column osteotomy techniques in 105 consecutive spinal deformity procedures. Spine (Phila Pa 1976) 2012;37:1198-210. [Crossref] [PubMed]
- Hassanzadeh H, Jain A, El Dafrawy MH, et al. Three-column osteotomies in the treatment of spinal deformity in adult patients 60 years old and older: outcome and complications. Spine (Phila Pa 1976) 2013;38:726-31. [Crossref] [PubMed]
- Yagi M, King AB, Boachie-Adjei O. Characterization of osteopenia/osteoporosis in adult scoliosis: does bone density affect surgical outcome? Spine (Phila Pa 1976) 2011;36:1652-7. [Crossref] [PubMed]
- Bhagat S, Vozar V, Lutchman L, et al. Morbidity and mortality in adult spinal deformity surgery: Norwich Spinal Unit experience. Eur Spine J 2013;22 Suppl 1:S42-6. [Crossref] [PubMed]
- Hu SS. Internal fixation in the osteoporotic spine. Spine (Phila Pa 1976) 1997;22:43S-8S. [Crossref] [PubMed]
- Pappou IP, Girardi FP, Sandhu HS, et al. Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis. Spine (Phila Pa 1976) 2006;31:1614-20. [Crossref] [PubMed]
- Tomé-Bermejo F, Piñera AR, Alvarez L. Osteoporosis and the Management of Spinal Degenerative Disease (II). Arch Bone Jt Surg 2017;5:363-74. [PubMed]
- Fu KM, Rhagavan P, Shaffrey CI, et al. Prevalence, Severity, and Impact of Foraminal and Canal Stenosis Among Adults With Degenerative Scoliosis. Neurosurgery 2011;69:1181-7. [Crossref] [PubMed]
- Ebata S, Takahashi J, Hasegawa T, et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J Bone Joint Surg Am. 2017;99:365-72. [Crossref] [PubMed]
- Gupta A, Cha T, Schwab J, et al. Osteoporosis Increases the Likelihood of Revision Surgery Following a Long Spinal Fusion for Adult Spinal Deformity. Spine J 2021;21:134-40. [Crossref] [PubMed]
- Armas LA, Recker RR. Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am 2012;41:475-86. [Crossref] [PubMed]
- Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the Women's Health Initiative. Semin Reprod Med 2014;32:454-62. [Crossref] [PubMed]
- Aspray TJ, Hill TR. Osteoporosis and the Ageing Skeleton. Subcell Biochem 2019;91:453-76. [Crossref] [PubMed]
- Gupta A, Upadhyaya S, Patel A, et al. DEXA sensitivity analysis in patients with adult spinal deformity. Spine J 2020;20:174-80. [Crossref] [PubMed]
- Baim S, Binkley N, Bilezikian JP, et al. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom 2008;11:75-91. [Crossref] [PubMed]
- Roh YH, Lee ES, Ahn J, et al. Factors affecting willingness to get assessed and treated for osteoporosis. Osteoporos Int 2019;30:1395-401. [Crossref] [PubMed]
- Augat P, Weyand D, Panzer S, et al. Osteoporosis prevalence and fracture characteristics in elderly female patients with fractures. Arch Orthop Trauma Surg 2010;130:1405-10. [Crossref] [PubMed]
- Freedman KB, Kaplan FS, Bilker WB, et al. Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am. 2000;82:1063-70. [Crossref] [PubMed]
- Gong HS, Oh WS, Chung MS, et al. Patients with wrist fractures are less likely to be evaluated and managed for osteoporosis. J Bone Joint Surg Am. 2009;91:2376-80. [Crossref] [PubMed]
- Le QA, Hay JW, Becker R, et al. Cost-effectiveness Analysis of Sequential Treatment of Abaloparatide Followed by Alendronate Versus Teriparatide Followed by Alendronate in Postmenopausal Women With Osteoporosis in the United States. Ann Pharmacother. 2019;53:134-43. [Crossref] [PubMed]
- Sahbani K, Cardozo CP, Bauman WA, et al. Abaloparatide exhibits greater osteoanabolic response and higher cAMP stimulation and β-arrestin recruitment than teriparatide. Physiol Rep 2019;7:e14225. [Crossref] [PubMed]
- Reginster JY, Hattersley G, Williams GC, et al. Abaloparatide is an Effective Treatment Option for Postmenopausal Osteoporosis: Review of the Number Needed to Treat Compared with Teriparatide. Calcif Tissue Int 2018;103:540-5. [Crossref] [PubMed]
- Eastell R, Mitlak BH, Wang Y, et al. Bone turnover markers to explain changes in lumbar spine BMD with abaloparatide and teriparatide: results from ACTIVE. Osteoporos Int 2019;30:667-73. [Crossref] [PubMed]
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434-41. [Crossref] [PubMed]
- Oswald AJ, Berg K, Ralston SH, et al. Long-Term Effects of Teriparatide Followed by Antiresorptive Therapy on Clinical Outcomes in Patients with Severe Spinal Osteoporosis. Calcif Tissue Int 2019;105:148-55. [Crossref] [PubMed]



