
Robotic navigation system utilization for percutaneous sacroiliac screw placement: surgical setup and technique
Introduction
The sacroiliac joint (SIJ) is a diarthrodial, synovial joint implicated as a source of chronic leg and lower back pain (LBP) (1). Sacroiliac joint dysfunction (SIJD) is involved in 13–30% of all patients with chronic LBP (2). The actual prevalence is likely underreported, as SIJD is often overlooked due to overlapping symptoms with other potential etiologies.
When attempts at conservative management of SIJD fail, surgical management can be considered. Surgical management of SIJD typically involves SIJ arthrodesis, with open techniques first described in the early 1900s (3,4). Minimally invasive surgical (MIS) techniques were described in the 2000s, and studies demonstrate that MIS SIJ fusion is superior to non-operative measures in regards to improving pain, function, and quality of life (5), up to at least 5 years postoperatively (6). MIS techniques also report decreased blood loss, surgical time, infections, neurovascular injury, and length of stay (7). Traditionally, intraoperative fluoroscopy was used to aid in implant placement. However, with growing emphasis on MIS, computed tomography (CT) navigation and robotic technology has been employed to improve efficiency and efficacy (8). In pedicle screw placement, robotic assistance has been shown to improve accuracy (9), decrease radiation exposure (10), reduce overall surgical and screw placement time (11), and allow for larger pedicle screws with reduced rate of breaches (11). MIS SIJ fusion using intraoperative navigation has been shown to be effective, efficient, and allow for accurate implant placement (8).
Surgical technique for robotic SIJ fusion has not been previously described. The purpose of this article is to provide a detailed workflow for the use of intraoperative robotic navigation for SIJ fusion, with a focus on clinical applications. Ourselves (12,13) and others (11) have recently described in depth how navigation technologies work, so that will not be the focus of this review. Similarly, the purpose of this paper is not to compare navigated vs. free-hand SIJ fusion, but rather to describe our experience and techniques to assist those interested in using this technology. We will discuss use of the Medtronic O-arm and StealthStation (Medtronic, Minneapolis, MN, USA), and the Globus ExcelsiusGPS (Globus Medical, Inc., Audubon, PA, USA) robotic navigation system, as the senior authors are most familiar with these systems. This review does not claim superiority of any particular brand or device, the discussion is intended to be applicable to all systems, and the surgeon is ultimately responsible for choosing which to use. We emphasize that intraoperative navigation and robotic guidance is not a substitute for knowledge of pertinent anatomy. These technologies are designed to assist and augment the skills of the surgeon, not replace them. Navigation alone should not be solely relied on, as this can lead to potential harm of the patient.
Method for utilizing robotic navigation
Room set up
A large operative theatre is preferred for the case, as this helps to ensure efficiency and maintenance of a safe and sterile environment given the use of multiple large devices, including C-arm, O-arm, and the robotic system.
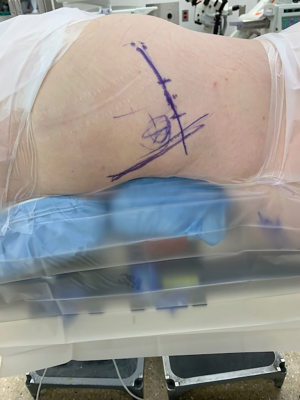
Neuromonitoring leads are placed after induction of anesthesia, allowing for electromyography (EMG) monitoring during the case, particularly of the L5 nerve root. The patient is positioned prone on a spine table. Care is taken to position the patients’ head and neck in a neutral position, and to pad all bony prominences. Palpation and C-arm fluoroscopy are used to identify landmarks. On the operative side, lines are drawn along the posterior cortical wall of the sacrum, and along the iliac cortical density, to estimate the anticipated incision location (Figure 1). The contralateral posterior superior iliac spine (PSIS) is marked, the utility of which is described below. The C-arm is removed from the room at this time.
Navigation preparation and image acquisition
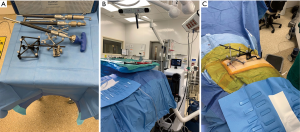
Prior to using reference frames and the navigated drill and screwdriver (Figure 2A), these tools are registered to the navigation station. A sensory camera is placed at the foot of the bed (Figure 2B), which allows for unencumbered tracking of the navigated instruments. A 1-centimeter (cm) skin incision is made over the contralateral PSIS and a tracking pin is docked into this, allowing for attachment of a passive navigation frame. An intraoperative computed tomography fixture (ICT) is then attached to this passive frame, which allows for the images obtained with the O-arm to automatically sync with the robotic navigation station (Figure 2C). The O-arm is brought in and an intraoperative spin is obtained.
SI screw templating
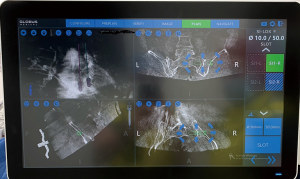
The robotic navigation station allows for templating of the desired sacroiliac screws’ length, width and trajectory via a touchscreen using three-dimensional (3D) imaging in the sagittal, axial and coronal planes (Figure 3). The authors’ preference is to template in the sagittal plane initially, followed by axial and coronal views, to ensure the screw is properly placed within the SI joint and sacral body. It is important to confirm that the screw trajectory does not violate the sacral foramina or canal, or travel above the iliac cortical density. Once satisfied with the screw template, this plan is saved within the robotic navigation system. If a multi-level construct is desired, the surgeon is able to select the next surgical level on the station, and repeat the process. Additionally, the navigation station projects skin incision locations of templated screws, so making minor adjustments in trajectory often makes it possible to make the screw entry points more convergent, thus performing a multilevel procedure through one small incision, rather than two.
SI screw placement
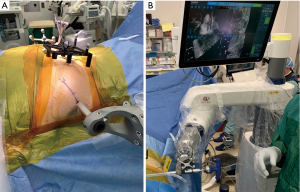
The robot is brought into the field and the desired level, i.e., S1, is selected on the navigation station. This prompts the robot arm to navigate to the planned start point on the patient, and this location is confirmed with our preoperative fluoroscopic estimated incision markings (Figure 4A). A small, approximately 1–2 cm incision through skin and fascia is made using a scalpel designed for use through the robotic arm. The trajectory is then drilled, and the SI screw is placed through the guidance of the robotic arm (Figure 4B). The robot guides the path of the drill and screw, preventing excessive skiving or deviation from the templated paths. The surgeon is able to watch on the navigation screen during drilling and screw insertion to ensure correct trajectory and length on multiple views (Figure 4B). When the screw reaches the correct templated location, the navigation system provides check marks to confirm this. If performing multi-level surgery, the surgeon would then select the next desired level, and repeat the process. Once all screws are placed, it is our preference to perform a final EMG test and intraoperative O-arm spin. This allows confirmation that the nerve roots have not been injured and all screws have been placed in safe positions, prior to leaving the operative suite.
Summary and case examples
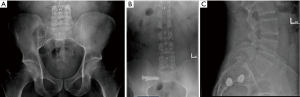
A 42-year-old female with a history of L5–S1 fusion presented with left buttock pain that radiated down her posterior thigh. Examination was notable for pain with palpation of the left SI joint, as well as pain in the left SI joint region with left hip flexion, abduction and external rotation (FABER). Imaging revealed her previous hardware without evidence of complications, as well as arthritic changes at the bilateral SI joints (Figure 5A). She was referred for a left SIJ injection, which transiently provided 100% relief of symptoms. She underwent a left SI fusion with screws at S1 and S2. She was discharged home the day of surgery, and at her 2-week postoperative appointment, her pain was completely resolved, and post-operative imaging confirmed well placed screws (Figure 5B,C).

A 31-year-old male laborer presented with right sided LBP, as well as right buttock, groin and thigh pain with paresthesias. Exam revealed right SI joint tenderness, and positive FABER. Imaging revealed a grade 1 L5–S1 isthmic spondylolisthesis without instability, and bilateral SI joint degeneration (Figure 6A). A right SI joint injection provided transient, but complete relief of his pain and paresthesias. Therefore, he underwent right SI joint fusion with screws at S1 and S2, and was discharged home on the day of surgery. At his 3-week postoperative appointment, his right sided symptoms were greatly improved and his paresthesias were completely resolved. Post-operative imaging confirmed well placed screws (Figure 6B,C).
Discussion
The SIJ is a common source of significant pain and discomfort in the lower back or legs (1,2). While a good history, physical examination, and radiographs are important in the workup, SIJ injection is the gold standard for diagnosis. Some etiologies of SIJD include fracture, soft tissue injury, sacroiliitis due to osteoarthritis or spondyloarthropathies, infection, scoliosis, and malignancy. Conservative treatment regimens for SIJD include physical therapy, manipulation of the joint, and anti-inflammatory medications. More invasive options include steroid injections and radiofrequency denervation. If conservative measures fail, surgical intervention is warranted. We generally aim for an 80% symptom improvement rate after steroid injection prior to surgical consideration.
MIS SIJ fusion is becoming more popular with the increased availability of intraoperative image-guided navigation and robotic systems. It is the authors preference to perform all of our SIJ fusion procedures, both routine or complex, using intraoperative robotic navigation. We would like to emphasize that SIJ fusions do not have to be done with robotic navigation. However, in our experience, when performing SIJ fusion using our described technique, we have found that utilizing this technology has improved our operative time, blood loss, accuracy, and ultimately patient outcomes.
Lastly, cost considerations should be taken into account when discussing robotic navigation technologies. The initial cost for robotic systems can vary widely, with prices ranging from $550,000 to $2 million, with annual maintenance fees around 10% of this initial cost (14,15). Despite these high initial costs, the literature shows that robotic systems are typically cost-effective in the long-term due to the fact that operative times, revision surgeries, length of stay in hospital, and infections are typically reduced by utilization of this technology (14,15). In a study comparing results of robotic vs. non-robotic thoracolumbar fusions in 557 patients, Menger et al. demonstrated a 1-year cost-savings of $608,546 by using robotic navigation, attributed to the above reasons (15).
Limitations
Although using intraoperative navigation and robotic instrumentation for placement of sacroiliac screws is not a necessity, it has the potential to be a valuable tool for the spine surgeon and patients. Further research is needed to elucidate the outcomes of robotic vs. non-robotic instrumented SI screws, but in the authors’ experience, it has been a useful and effective tool, reducing our operative time, blood loss, fluoroscopic use, and screw accuracy. Additionally, utilization of the robot is likely to be cost-effective in the long-term.
We would again like to emphasize that these technologies are not meant to replace knowledge of operative anatomy or steps. The surgeon should not become complacent or overly-confident when using navigation or robotics. Anatomical landmarks and knowledge should be continuously used throughout to verify accuracy and provide safeguard against possible malfunction or inaccuracy of these systems.
Conclusions
Robotics and navigation in spine surgery are exciting and growing technologies that have the potential to offer the surgeon many new perioperative advantages. The described technique for robotic navigated SIJ fusion provided in this paper is meant to serve as a guide to help the efficiency and safety of others using this technology. We have found our method to be reproducible, safe, and beneficial to our patients.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at http://dx.doi.org/10.21037/jss-20-681
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-681). Dr. RDP reports personal fees and other from Globus, outside the submitted work; Dr. ISA reports grants from Orthofix, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg 2005;101:1440-53. [Crossref] [PubMed]
- Cohen SP, Chen Y, Neufeld NJ. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother 2013;13:99-116. [Crossref] [PubMed]
- Verrall J. Discussion on the Diagnosis and Treatment of Affections of the Sacro-iliac Joint. Proc R Soc Med 1925;18:24. [Crossref] [PubMed]
- Ledonio CG, Polly DW Jr, Swiontkowski MF. Minimally invasive versus open sacroiliac joint fusion: are they similarly safe and effective? Clin Orthop Relat Res 2014;472:1831-8. [Crossref] [PubMed]
- Whang P, Cher D, Polly D, et al. Sacroiliac Joint Fusion Using Triangular Titanium Implants vs. Non-Surgical Management: Six-Month Outcomes from a Prospective Randomized Controlled Trial. Int J Spine Surg 2015;9:6. [Crossref] [PubMed]
- Rudolf L, Capobianco R. Five-year clinical and radiographic outcomes after minimally invasive sacroiliac joint fusion using triangular implants. Open Orthop J 2014;8:375-83. [Crossref] [PubMed]
- Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res 2013;7:14. [Crossref] [PubMed]
- Lee DJ, Kim SB, Rosenthal P, et al. Stereotactic guidance for navigated percutaneous sacroiliac joint fusion. J Biomed Res 2016;30:162-7. [PubMed]
- Perdomo-Pantoja A, Ishida W, Zygourakis C, et al. Accuracy of Current Techniques for Placement of Pedicle Screws in the Spine: A Comprehensive Systematic Review and Meta-Analysis of 51,161 Screws. World Neurosurg 2019;126:664-678.e3. [Crossref] [PubMed]
- Villard J, Ryang YM, Demetriades AK, et al. Radiation exposure to the surgeon and the patient during posterior lumbar spinal instrumentation: a prospective randomized comparison of navigated versus non-navigated freehand techniques. Spine (Phila Pa 1976) 2014;39:1004-9. [Crossref] [PubMed]
- Vaccaro AR, Harris JA, Hussain MM, et al. Assessment of Surgical Procedural Time, Pedicle Screw Accuracy, and Clinician Radiation Exposure of a Novel Robotic Navigation System Compared With Conventional Open and Percutaneous Freehand Techniques: A Cadaveric Investigation. Global Spine J 2020;10:814-25. [Crossref] [PubMed]
- Butt BB, Piche J, Gagnet P, et al. Stereotactic navigation in anterior cervical spine surgery: surgical setup and technique. J Spine Surg 2020;6:598-605. [Crossref] [PubMed]
- Wallace N, Schaffer NE, Freedman BA, et al. Computer-assisted navigation in complex cervical spine surgery: tips and tricks. J Spine Surg 2020;6:136-44. [Crossref] [PubMed]
- D'Souza M, Gendreau J, Feng A, et al. Robotic-Assisted Spine Surgery: History, Efficacy, Cost, And Future Trends. Robot Surg 2019;6:9-23. [Crossref] [PubMed]
- Menger RP, Savardekar AR, Farokhi F, et al. A Cost-Effectiveness Analysis of the Integration of Robotic Spine Technology in Spine Surgery. Neurospine 2018;15:216-24. [Crossref] [PubMed]


