
Esophagopharyngeal perforation and prevertebral abscess after anterior cervical discectomy and fusion: a case report
Introduction
Anterior cervical discectomy and fusion (ACDF) represents one of the most commonly performed spine surgeries globally. The United States alone averages almost 137,000 of these procedures yearly with successful outcomes (1). While the risk of complications is relatively rare, they can carry high morbidity, especially when they require reoperation. Among these complications includes wound infections, which have a documented risk of 0.1% to 1.6% in the literature (2). Most of these infections tend to be superficial and occur early in the perioperative period due to direct inoculation, hematogenous seeding, or poor wound care. However, delayed deep infections, occurring, greater than 2 months from surgery, carry a more significant burden leading to more morbidity and mortality, and can commonly be caused by esophageal perforation leading to seeding of oropharyngeal flora in the deep prevertebral space (3).
Esophageal injury most commonly occurs during the initial operation and can go unrecognized. An injury due to retraction usually leads to dysphagia which is the most common complaint after anterior approach to the cervical spine (3,4). However, a significantly rarer and more feared complication is actual perforation and violation of the esophageal tissue, which can lead to local soft tissue swelling, delayed deep infection with hardware failure, pseudarthrosis, osteomyelitis/discitis, sepsis, and in the worst cases death (5-9). Due to the rarity of this complication, with an incidence reported between 0.3–0.9%, a gold standard of management is yet to be described. We present a unique case of a delayed presentation of recurrent esophagopharyngeal perforation leading to a large prevertebral abscess, osteomyelitis, and implant failure necessitating multiple procedures and complex soft tissue reconstruction.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-646).
Case presentation
A healthy 47-year-old female presented to our institution status post a C4–C7 ACDF performed at an outside institution 9 months prior for cervical myelopathy. After her initial procedure, the patient complained of mild dysphagia, but tolerated her diet and was discharged home on postoperative day 2 without any adverse events. The patient was followed routinely with postoperative visits including serial radiographs, but had continued complaints of dysphagia. The patient was seen by otolaryngology and ultimately underwent an esophagoscopy 4 weeks prior to arriving to our institution, which demonstrated no esophageal injury. Of note the patient did not have any comorbidities that would predispose her to infections and denied all tobacco use, intravenous (IV) drug abuse, or use of steroids.
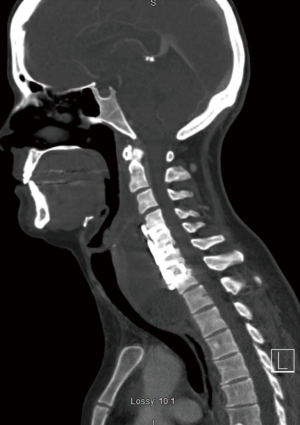
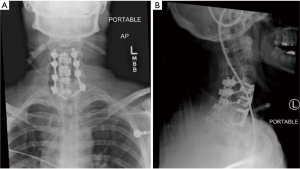
Seven days prior to her arrival to our institution, the patient started experiencing increased dysphagia, neck pain and swelling, fevers, and right upper extremity radicular pain and numbness. Upon arrival to our emergency room, the patient was febrile to 102 degrees, had a white blood cell count of 17.3, erythrocyte sedimentation rate (ESR) of 47, and C-reactive protein (CRP) of 21.4. A computed tomography (CT) and magnetic resonance imaging (MRI) with contrast of the neck was obtained which demonstrated a 35 mm × 59 mm × 96 mm large retropharyngeal prevertebral rim-enhancing mass extending from C3–C4 to the upper thoracic spine displacing the esophagus and trachea anteriorly (Figure 1). The patient was taken to the operating room for an incision and drainage of the abscess utilizing the previous anterior surgical incision. A large purulent cavity was encountered where 400 cc of pus was evacuated, cultured, and thoroughly irrigated. The fusion site was inspected which demonstrated a solid fusion at the levels of C4–C6, and therefore the prior interbody cages were retained and fusion hardware at these levels were reinserted. Non-union with instability and a loose implant was noted at the C6–C7 level with obvious osteomyelitis in the interbody space. With resection of the PLL the epidural space was carefully evacuated and irrigated. A partial (50%) corpectomy of the C6 body was performed to achieve complete decompression and address the possible osteomyelitis, and a new polyetheretherketone (PEEK) spacer cage and anterior instrumentation with plate and screws was performed. Throughout the procedure, no defect or scars in the mucosa or evidence of esophageal defect, tear, or diverticulum was found. Due to the poor bone stock and fixation anteriorly with a three-level construct, as well as the anticipation that a complete removal of hardware anteriorly may be required, a staged posterior decompression and fusion was planned. The next day a posterior spinal instrumented fusion from C4–T1 was performed, with laminectomy and decompression at the C6–C7 level and bilateral lamino-foramenotomies at C4–C5 level (Figure 2). A postoperative barium esophagogram demonstrated no extravasation of contrast or evidence of esophageal leak. A postoperative repeat MRI demonstrated resolution of the epidural and prevertebral fluid collection and abscess.
Unfortunately, the patient continued to have persistent purulent wound drainage and neck discomfort requiring a repeat serial irrigation and debridement through the anterior incision. An intraoperative Indigo carmine dye was inserted through the orogastric (OG) tube to look for any obvious signs of esophageal leakage. A 5 mm diameter area of blue dye was seen around the level of C6, which we surmised represented a partial thickness defect of the esophageal wall. An intraoperative ear nose and throat consult was placed, but no further intervention was recommended.
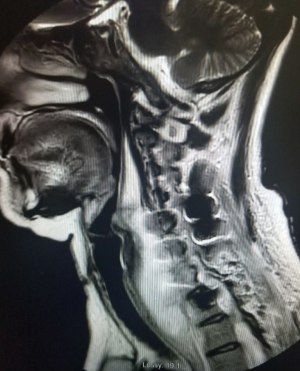
Over the next week, the patient had worsening symptoms and continued difficulty swallowing with white purulent drainage though the hemovac drain. Advanced imaging again demonstrated a large residual prevertebral collection necessitating another serial irrigation and debridement. At this juncture, all the anterior hardware, aside from the interbody cages, was removed. A few days after this procedure, the patient again complained of worsening anterior throat pain and dysphagia and 30 cc of purulent material was aspirated from the surgical site. A repeat MRI demonstrated decreased mediastinal and prevertebral fluid collection, but did demonstrated possible osteomyelitis at the levels of the C6 and C7 vertebral bodies (Figure 3). The patient again returned to the operating room in conjunction with an ear nose and throat surgeon. A nasogastric (NG) tube was passed, and the ENT surgeon confirmed that there was no esophageal or pharyngeal mucosal defect or rupture. A completion of the prior C6 and a new full C7 corpectomy was performed and an expandable cage was applied in conjunction with the application of antibiotic impregnated calcium sulfate for local elution of antibiotics (Figure 4). The patient was discharged on 6 weeks of IV antibiotics through a peripherally inserted central catheter (PICC) line.
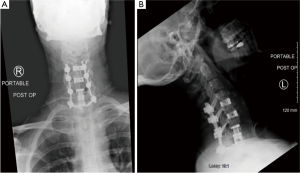
Regrettably, the patient was readmitted to the hospital 3 weeks later due to poor wound healing with drainage of food particles from the incision site. A repeat barium esophagram demonstrated extravasation of the dye material from the esophagus at the level of C7 with a fistula communicating to the skin surface (Figure 5). The patient was taken back to the operating room by ENT who performed a direct laryngoscopy that demonstrating a right piriformis tear leading to esophageal and pharyngeal injury and fistula. It was noted that there was significant scarring of the pharyngeal and esophageal mucosa adjacent to the expandable cage. The piriformis tear was repaired primarily with 3-0 and 4-0 vicryl and a repeat laryngoscopy and hydrogen peroxide leak test were performed which demonstrated no extravasation. The decision was made to perform a sternocleidomastoid muscle (SCM) flap on the right side to provide healthy tissue between the pharynx and hardware.
Ten days later the patient was found to have orange drainage from her surgical site with increasing neck discomfort. The patient had just resumed per os (PO) intake. Repeat advanced imaging demonstrated a rim enhancing pocket suspicious for an abscess at the level of the C5–C6 hardware and extravasation of contrast with perforation at the level of the previous fistula repair. Patient was given an open gastrotomy tube for post-operative nutrition and then underwent direct laryngoscopy and closure of a 1.5 cm defect located at the posterior pharyngeal wall, revision of SCM flap, and injection of botox to the cricopharyngeus muscle and bilateral submandibular and parotid glands to decrease the amount of secretions. The patient also received an anterolateral thigh free flap for definitive soft tissue coverage. During this procedure, the interbody cage at the level of C5–C6 was found to be protruding about 5 mm anterior, and was thought to be affecting the healing of the esophageal and pharyngeal repair. As such, the interbody cage was removed and replaced with a tantalum cage with a smaller footprint. The patient is now 6 months status post her final procedure. Patient is currently infection free, has no symptoms of dysphagia, no wound issues, or neurological deficits. No further surgical intervention is planned.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
ACDF is the most commonly performed cervical spine surgery in the treatment of degenerative disc disease, traumatic cervical diseases, or cervical spondylosis. This procedure has enjoyed excellent outcomes, with a relatively small complication rate (1). The reported complication profile of ACDF, from most to least common, includes dysphagia, post-operative hematoma, exacerbation of myelopathy or radiculopathy, recurrent laryngeal nerve palsy, cerebrospinal fluid (CSF) leak, wound infection, hematoma, laryngeal nerve palsy, CSF leak, Horner’s syndrome, respiratory insufficiency, esophageal perforation, and instrument failure (2,4,10,11).
While dysphagia is by far the most common complaint after ACDF, most of these can be classified as mild as they tend to resolve spontaneously within 1 week to 2 months and are due to temporary retraction type injuries (3,4). On the other hand, severe dysphagia or dysphagia that lingers for an extended period must be taken more seriously and can represent more morbid complications. They can be indicative of esophageal or pharyngeal perforations, which have been reported to have an incidence of 0.3–0.9% and to have mortalities rates up to 20% or even 50% if treatment is delayed (5,12). They can lead to devastating complications such as prevertebral abscess formation, osteomyelitis, mediastinitis, aspiration of extruded instrumentation, and death from septicemia (6,7,13-18). Due to the paucity of literature on delayed prevertebral abscess formation with or without pharyngoesophageal perforation, the appropriate management of this complication is still not well understood. A current literature review found less than 10 case reports of late prevertebral abscess formation, none of which required this number and complexity of revision procedures. Therefore, this case reinforces critical learning points that could provide clarity on future treatment protocols for this rare complication.
The work-up of dysphagia should begin with a thorough history and physical examination. Serial cervical spine radiographs should be examined to critically evaluate the location of all hardware for migration or prominence and to look for prevertebral space edema or emphysema. While some edema and swelling in the perioperative period is normal, late dysphagia should not have any associated swelling and warrants further investigation. Symptoms such as neck pain and swelling, fever, and elevated infectious labs such as ESR, CRP, and elevated white blood cell count should prompt urgent advanced imaging. A CT and or MRI of the neck with and without contrast should be obtained to rule out prevertebral abscess and or osteomyelitis. Finally, an ENT consult should be placed urgently to evaluate for esophageal/pharyngeal perforation, most commonly using a barium esophagogram (6,13,19-21). This will also evaluate for the rare Zenker’s diverticulum, which has been associated with late dysphagia after ACDF (22). In our case, the patient did undergo extensive workup of her dysphagia even before entering our institution. Although multiple esophagograms failed to show any significant perforation, an intraoperative dye test did demonstrate a partial thickness esophageal injury. We hypothesize that this could have represented a clinically latent full thickness perforation that occurred at some point during the perioperative period that attempted to heal. This tissue is most certainly weaker than native tissue and could explain the patient’s recurrent infections and fistulas. As such, even presumed partial thickness injuries to the esophagopharyngeal anatomy should be critically analyzed, with a low threshold to intervene. As supported by this case, early direct visualization of the mucosa through intraoperative dye testing or even laryngoscopy may be beneficial and should be considered when symptoms persist even if esophagrams are negative.
Early esophageal perforation after ACDF can be caused through several different mechanisms. Most commonly this is due to iatrogenic damage secondary to retractor placement, inadvertent perforation with electrocautery or sharp instruments during dissection or drilling, or even traumatic intubation (5,11,12,21). While full-fledged perforations are rare, microtrauma and incomplete injuries to the esophagus are probably more common than reported, and can over time lead to more catastrophic full tissue disruptions—as was the case in this patient. Delayed pharyngoesophageal perforations are most commonly caused by microtrauma secondary to irritation by hardware or bone against the posterior wall of the esophagus (7,17,18). Some studies have suggested that lower profile constructs may be associated with lower rates of dysphagia (23). Delayed hardware failure with screw migration has also been found to be associated with pharyngoesophageal injury (13). In extremely rare cases, actual extrusion of the instrumentation has also been reported (14,16). Although not relevant to our specific case, intraoperative retropharyngeal steroid use has also been described as a possible cause of late esophageal perforation in certain case studies (18). While the suggested mechanisms for both pharyngeal and esophageal perforations are the same, pharyngeal perforations are much less common and rarely reported in the literature. We deduced that one of the major culprits to the esophageal injury in our case was due to some mild prominence of the anterior hardware irritating an already partially damaged mucosa. Removal of all anterior hardware as early as possible or use of implants with a smaller footprint may be prudent.
Finally, due to how rare esophageal perforations are after ACDF, there is not an abundance of literature describing the optimal management of soft-tissue defects in this area. It has been shown that primary closure is rarely adequate for definitive closure after esophageal perforation, as the native tissue is usually tenuous after undergoing multiple revision procedures (24-26). We would argue that all leaks after ACDF surgery should be addressed both with primary repair and a vascularized tissue. The SCM flap is an excellent option, due to its proximity to the injury, its ease of mobilization, and minimal donor site morbidity. The reason for early use of this flap is to create a buttress between the scarred bone of the cervical vertebrae and the cervical esophagus while also eliminating dead space, improving antibiotic delivery, and shortening the time to recovery. Navarro et al. was one of the first authors to describe the role of SCM flap for esophageal fistula repair follow anterior cervical surgery (27). Since then, multiple authors have further expanded on the indications, and more importantly, the limitations of the SCM flap. While the SCM flap has been demonstrated to provide reliable outcomes in esophageal defects follow anterior cervical surgery, studies have commented that the flap tip may be too small for larger soft-tissue defects (26-28). For larger defects, many authors have advocated for free tissue transfers, such as an omentum, pectoralis, or anterolateral thigh free flap—citing their larger size and better vasculature (22,24,25,29-32). In our case, although an SCM flap was attempted, continued hardware irritation and the large soft-tissue defect created from multiple surgeries and recurrent fistulas required the SCM flap needed to be augmented with an anterolateral thigh free flap. Involving and collaborating early with otolaryngology allows for immediate and definitive soft tissue closure and provides a critical analysis of any future issues and healing potential. This should lead to a decreased necessity for multiple procedures.
This was a unique case of a delayed presentation of both esophageal and pharyngeal perforation leading to a large prevertebral abscess, osteomyelitis, and implant failure necessitating multiple procedures and complex soft tissue reconstruction. Dysphagia in the late postoperative setting should always be evaluated carefully and thoroughly for any esophageal perforation and deep infection. As exemplified in this case, even partial thickness injuries to the esophageal-pharyngeal anatomy can lead to catastrophic complications over time. Safe and early removal of all hardware anteriorly to avoid continued irritation of the esophagopharyngeal mucosa should be prioritized. If anterior hardware is necessary for stability, implants with the smallest footprint should be utilized. Early intraoperative collaboration with ear nose and throat colleagues should be a priority, and can provide timely diagnostic and therapeutic interventions. Complex closure with a free flap was shown to be an effective way to provide successful definitive soft tissue coverage.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-646
Peer Review File: Available at http://dx.doi.org/10.21037/jss-20-646
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-646). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Epstein NE. A review of complication rates for anterior cervical diskectomy and fusion (ACDF). Surg Neurol Int 2019;10:100. [Crossref] [PubMed]
- Vaishnav AS, Saville P, McAnany S, et al. Predictive factors of postoperative dysphagia in single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2019;44:E400-7. [Crossref] [PubMed]
- Shriver MF, Lewis DJ, Kshettry VR, et al. Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Global Spine J 2017;7:95-103. [Crossref] [PubMed]
- Halani SH, Baum GR, Riley JP, et al. Esophageal perforation after anterior cervical spine surgery: a systematic review of the literature. J Neurosurg Spine 2016;25:285-91. [Crossref] [PubMed]
- Christiano LD, Goldstein IM. Late prevertebral abscess after anterior cervical fusion. Spine (Phila Pa 1976) 2011;36:E798-802. [Crossref] [PubMed]
- Yang SY, Lee SB, Cho KS. Delayed esophagus perforation after anterior cervical spine surgery. Korean J Neurotrauma 2015;11:191-4. [Crossref] [PubMed]
- Park MK, Cho DC, Bang WS, et al. Recurrent esophageal perforation after anterior cervical spine surgery: case report. Eur Spine J 2018;27:515-9. [Crossref] [PubMed]
- Chen YC, Zhang L, Li EN, et al. Late deep cervical infection after anterior cervical discectomy and fusion: a case report and literature review. BMC Musculoskelet Disord 2019;20:437. [Crossref] [PubMed]
- Helseth Ø, Lied B, Heskestad B, et al. Retrospective single-centre series of 1300 consecutive cases of outpatient cervical spine surgery: complications, hospital readmissions, and reoperations. Br J Neurosurg 2019;33:613-9. [Crossref] [PubMed]
- Yee TJ, Swong K, Park P. Complications of anterior cervical spine surgery: a systematic review of the literature. J Spine Surg 2020;6:302-22. [Crossref] [PubMed]
- Harman F, Kaptanoglu E, Hasturk AE. Esophageal perforation after anterior cervical surgery: a review of the literature for over half a century with a demonstrative case and a proposed novel algorithm. Eur Spine J 2016;25:2037-49. [Crossref] [PubMed]
- Vrouenraets BC, Been HD, Brouwer-Mladin R, et al. Esophageal perforation associated with cervical spine surgery: report of two cases and review of the literature. Dig Surg 2004;21:246-9. [Crossref] [PubMed]
- Gazzeri R, Tamorri M, Faiola A, et al. Delayed migration of a screw into the gastrointestinal tract after anterior cervical spine plating. Spine (Phila Pa 1976) 2008;33:E268-71. [Crossref] [PubMed]
- Dakwar E, Uribe JS, Padhya TA, et al. Management of delayed esophageal perforations after anterior cervical spinal surgery. J Neurosurg Spine 2009;11:320-5. [Crossref] [PubMed]
- Cagli S, Isik HS, Zileli M. Cervical screw missing secondary to delayed esophageal fistula: case report. Turk Neurosurg 2009;19:437-40. [PubMed]
- Kim SJ, Ju CI, Kim DM, et al. Delayed esophageal perforation after cervical spine plating. Korean J Spine 2013;10:174-6. [Crossref] [PubMed]
- Lee SH, Mesfin A, Riew KD. Delayed esophageal perforation after anterior cervical fusion and retropharyngeal steroid use: a report of two cases. Spine J 2015;15:e75-80. [Crossref] [PubMed]
- Witwer BP, Resnick DK. Delayed esophageal injury without instrumentation failure: complication of anterior cervical instrumentation. J Spinal Disord Tech 2003;16:519-23. [Crossref] [PubMed]
- Solerio D, Ruffini E, Gargiulo G, et al. Successful surgical management of a delayed pharyngo-esophageal perforation after anterior cervical spine plating. Eur Spine J 2008;17:S280-4. [Crossref] [PubMed]
- Kang MS, Kim KH, Park JY, et al. Management of esophageal and pharyngeal perforation as complications of anterior cervical spine surgery. World Neurosurg 2017;102:275-83. [Crossref] [PubMed]
- von Rahden BH, Stein HJ, Scherer MA. Late hypopharyngo-esophageal perforation after cervical spine surgery: proposal of a therapeutic strategy. Eur Spine J 2005;14:880-6. [Crossref] [PubMed]
- Lee MJ, Bazaz R, Furey CG, et al. Influence of anterior cervical plate design on Dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech 2005;18:406-9. [Crossref] [PubMed]
- Pichler W, Maier A, Rappl T, et al. Delayed hypopharyngeal and esophageal perforation after anterior spinal fusion: primary repair reinforced by pedicled pectoralis major flap. Spine (Phila Pa 1976) 2006;31:E268-70. [Crossref] [PubMed]
- Hanwright PJ, Purnell CA, Dumanian GA. Flap reconstruction for esophageal perforation complicating anterior cervical spinal fusion: an 18-year experience. Plast Reconstr Surg Glob Open 2015;3:e400 [Crossref] [PubMed]
- Ghirelli M, Molinari G, Rosini M, et al. Pharyngo-esophageal perforations after anterior cervical spine surgery: management and outcomes. World Neurosurg 2020;139:e463-73. [Crossref] [PubMed]
- Navarro R, Javahery R, Eismont F, et al. The role of the sternocleidomastoid muscle flap for esophageal fistula repair in anterior cervical spine surgery. Spine (Phila Pa 1976) 2005;30:E617-22. [Crossref] [PubMed]
- Benazzo M, Spasiano R, Bertino G, et al. Sternocleidomastoid muscle flap in esophageal perforation repair after cervical spine surgery: concepts, techniques, and personal experience. J Spinal Disord Tech 2008;21:597-605. [Crossref] [PubMed]
- Wierzbicka M, Bartochowska A, Banaszewski J, et al. Cervical oesophageal and hypopharyngeal perforations after anterior cervical spine surgery salvaged with regional and free flaps. Neurol Neurochir Pol 2013;47:43-8. [Crossref] [PubMed]
- Coelho R, Ekberg T, Svensson M, et al. Reconstruction of late esophagus perforation after anterior cervical spine fusion with an adipofascial anterolateral thigh free flap: a case report. Microsurgery 2017;37:684-8. [Crossref] [PubMed]
- Morita M, Matsumoto H, Shirakawa Y, et al. Flap reconstruction for esophageal perforation following anterior cervical plate fixation. Acta Med Okayama 2019;73:77-80. [PubMed]
- Gibson AW, Gobillot TA, Bass DI, et al. Case of esophageal perforation and repair with a supraclavicular artery island fascial flap 15 years after anterior spine surgery. World Neurosurg 2020;143:102-7. [Crossref] [PubMed]



