
Revisionary soft tissue reconstruction of posterior midline defects after spinal surgery—plastic reconstructive options including perforator flaps
Introduction
Soft tissue defects with exposure of spinal hardware represent a challenging complication for the multidisciplinary treatment team of orthopedic, neuro- as well as plastic surgeons. Normally, the human back is characterized by a notable surplus of soft tissues allowing multiple flaps such as muscle flaps and adipo-cutaneous rotation flaps. However, these “traditional” workhorses of plastic surgery have their limitations in a subset of patients with extensive defects or unfavorable wound location. Furthermore, surrounding scars, extended radiation fields, wound infections, previous surgeries and prominent implants may impair reconstructive attempts (1-3). The latissimus dorsi flap as well as the trapezius myocutaneous flap or sliding paraspinous muscle flaps are common treatment options for posterior trunk defects (4-6). However, muscle flaps in mobile patients may come along with considerable donor site morbidity such as loss of strength or function. Additionally, soft tissue defects after spinal surgery most-frequently occur in cases of multiple previous operations, after severe wound infection or previous radiotherapy. In these cases, the surrounding soft tissue is often compromised due to malperfusion, severe subcutaneous scarring and therefore local muscle advancement flaps may not allow stable defect reconstruction. Additionally, multiple multidirectional scaring due to prior skin incisions often proscribe traditional adipocutaneous flap rotation patterns or minimize flap sizes to useless dimensions. Therefore, defect closure with healthy tissue from unaffected and well-vascularized areas of the back are required. One therapeutic option can therefore be dorsal intercostal artery or lumbar artery perforator flaps that are more and more established as a technique of choice for dorsal midline defect closure (7,8). The physiological rationale behind these flaps is the angiosome, skin and subdermal tissue supplied by only one dominant perforator artery and vein (9). The territory of perfusion of each branch is located in an oblique manner from superior-medial to inferior-lateral parallel to the ribs (10). The flap may be rotated up to 180° to provide unscarred soft tissue to the defect site. In these cases, it is called propeller flap. However, in complex cases several different reconstructive techniques may be combined to sustainably reconstruct exposed spinal hardware. To date, there is no gold standard for the treatment of dorsal midline defects with exposed spinal hardware (11). The aim of the present study was to suggest a treatment algorithm for soft tissue coverage of these rare and highly complex defects in patients who have failed multiple previous reconstructive surgeries.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jss-20-688).
Methods
All patients treated between 2011 and 2015 with exposed spinal hardware after spinal surgery were evaluated retrospectively. The present analysis was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the governmental regional ethics committee (2019-14589). Due to the retrospective design of the present analysis written informed consent was not necessarily obtained from each patient. Cases were evaluated in means of patient’s age, pre-existing diseases, wound size, cause and defect location. Furthermore, major and minor complications, number of operations needed for complete wound closure as well as postoperative hospitalization were evaluated. In addition, subgroup analysis was assessed concerning different flaps used for defect closure regarding revision surgery needed (perforator flaps vs. conventional randomized flaps vs. combination of both flap entities). We assessed bacterial wound colonization and resistograms in wound samples taken intra-operatively. Therapeutic strategies and intra-operative decision-making were evaluated and a treatment regimen was deduced from the authors’ experiences. Preoperative variables were collected from medical records using a standardized data collection. Information regarding preoperative risk factors was derived from standardized and routinely recorded data as reported in the patient charts. Most patients were presented from other departments with impaired wound healing after spinal surgery and were treated with classical local advancement flaps without success. If patients had multiple pre-existing diseases or reduced general condition, nutritional status was assessed on admission and deficient nutrients were substituted prior to flap surgery to improve post-operative wound healing. During initial radical debridement bone and soft tissue samples were taken for histological and microbiological analyses. Subsequent debridements were completed and wounds were repeatedly treated with negative pressure wound therapy until clean and vital wounds were ensured (12). Preoperatively, all cases were discussed interdisciplinary with orthopedic and neuro-surgeons to determine the individual treatment algorithm. As standard, whenever possible spinal hardware was removed during initial debridement. Spinal hardware exchange was performed with flap reconstruction in the same surgical procedure. However, hardware replacement was impossible in a subset of patients and wound closure was planned in these cases accepting an increased risk of infection recurrence. In cases of bacterial colonization systemic antibiotic therapy based on the microbiological specimen and resistogram were applied. In cases of acute or chronic osteomyelitis proven in histopathology the antibiotic treatment was continued for at least 6 weeks postoperatively.
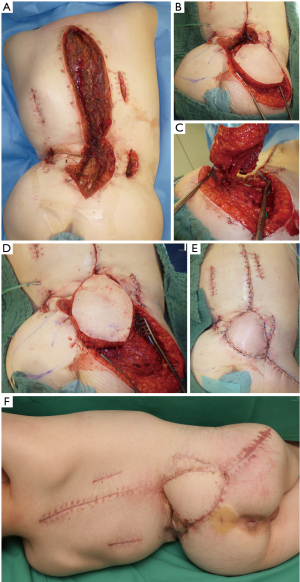
Most frequently, perforator flaps were used for reconstructive surgery. Flap dimensions and orientation are marked according to the estimated vascular territory, defect size and pre-operative Doppler or duplex ultrasound. With magnifying loops the vascular pedicle is identified and preserved (Figure 1). When a suitable vascular pedicle is adequately exposed skin incision is completed and the flap may be rotated up to 180° (Figure 2). If a propeller flap is technically unfeasible transfer may be achieved by YV-advancement. Random pattern flaps were applied if no adequate perforator was found. In larger defects a bilateral approach or a combination of different flap types may be necessary. In these cases, the authors clearly recommend to combine more than one flap to avoid partial flap loss due to excessive flap dimensions. In very large defects or severe surrounding scars a combination of different local flaps or free flaps may be considered. As standard all patients were placed into a pulsating air suspension bed postoperatively. The first three post-operative days the flap was regularly checked for arterial or venous congestion. Suction-drains were applied intra-operatively to allow hematoma drainage and to support adhesion of the flap to the wound bed and potential wound cavities.
Statistical analysis was performed using one-way ANOVA with a Bonferroni adjustment. Differences were defined as significant when P value <0.05.
Results
From 2011 to 2015, 18 patients with exposed spinal hardware were treated. Fourteen patients receiving 28 flaps were included in the present study (Table 1). In four patients wound closure was achieved after implant removal without flap surgery due to small defect sizes. Patients’ mean age was 51.1 years, ranging from 11 to 88 years. Most of the patients had multiple co-morbidities. Eight patients were classified as ASA 3 (American Society of Anesthesiologists). Five were classified as ASA 2 and only 1 as ASA 1. Thirteen patients (92.9%) underwent multiple prior operations and previous reconstructive attempts elsewhere. Reasons for the initial spinal surgery were: decompression of spinal canal stenosis (n=4), correction of a spina bifida (n=4), internal fixation of traumatic spine injuries (n=4) and oncologic resection (n=2). Defect sizes ranged from 6 to 525 cm2 with a mean size of 144 cm2. Defects were located over the lumbar spine (n=8), the cervical spine (n=2) and the thoracic spine (n=1). Extended longitudinal defects in three patients (21.4%) affected more than one of these areas.
Table 1
| Case No. | Sex | Age |
ASA- |
Time of flap surgery (minutes) | Hospital |
Defect |
Number of flaps needed for full coverage | Type of flap | Implant removal | Revision surgery | Wound complications | Surgical revision |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 26 | 2 | 192 | 56 | 260 | 3 | 3× perforator flap + split skin grafts | Implant exchange during flap surgery | 1 | Impaired wound healing due to distal flap mal perfusion | Flap readvancement |
| 2 | F | 11 | 3 | 289 | 16 | 70 | 3 | 1× perforator flap + 2× advancement flap | Implant exchange during flap surgery | 0 | – | – |
| 3 | F | 21 | 3 | 160 | 55 | 96 | 2 | 1× perforator flap + 1advancement flap (partially buried) | Subsequent partial implant removal due to local infection | 4 | Wound infection | 3× consecutive debridement+ negative pressure therapy, wound suture |
| 4 | M | 11 | 3 | 238 | 32 | 63 | 1 | 1× perforator flap (propeller) | Implant exchange during flap surgery | 0 | – | – |
| 5 | M | 82 | 3 | 202 | 36 | 162 | 1 | 1× pedicled latissimus dorsi-flap | Implant exchange during flap surgery | 1 | Wound infection | Flap elevation, wound irrigation and suture |
| 6 | F | 73 | 3 | 125 | 41 | 75 | 2 | 2× perforator flap | Implant removal during initial debridement | 0 | – | – |
| 7 | F | 73 | 3 | 165 | 60 | 117 | 1 | 1× perforator flap (partially buried) | No implant removal possible | 7 | Wound infection | 4× consecutive debridement + negative pressure therapy, 1× secondary suture, 2× split skin grafts |
| 8 | M | 62 | 1 | 120 | 14 | 6 | 1 | 1× randomized rotation flap | Implant removal during initial debridement | 0 | – | – |
| 9 | F | 55 | 2 | 130 | 18 | 10 | 2 | 2× advancement flap | Implant exchange during flap surgery | 0 | – | – |
| 10 | M | 66 | 2 | 77 | 13 | 33 | 2 | 2× randomized rotation flap | Implant removal during initial debridement | 0 | – | – |
| 11 | F | 25 | 3 | 480 | 30 | 525 | 4 | 1x perforator flap (propeller), 2× randomized rotation flap, 1x perforator flap, split skin grafts | Implant removal during initial debridement | 1 | Postoperative hematoma | Wound irrigation and suture |
| 12 | M | 72 | 2 | 336 | 28 | 300 | 1 | 1× free latissimus dorsi-flap | Implant removal during initial debridement | 0 | – | – |
| 13 | M | 51 | 2 | 237 | 66 | 200 | 3 | 2× perforator flap, 1× randomized rotation flap, split skin grafts | No implant removal possible | 4 | Wound infection | 2× debridement + negative pressure therapy 2× split skin grafts |
| 14 | M | 88 | 3 | 236 | 25 | 100 | 2 | 2× perforator flap (propeller, partially buried) | Implant removal during initial debridement | 0 | – | – |
| Mean | 7 F, 7 M | 51.1 | – | 213.4 | 35.0 | 144.1 | 2 | – | – | – | – | – |
ASA, American Society of Anesthesiologists; F, male; M, male.
After admission 7 patients (50.0%) underwent only one debridement prior to flap surgery, whereas the others needed more than one debridement to achieve clean wounds clinically and in microbiology specimen. Average time of spinal hardware exposure prior to admission was 48.8 days. In six patients (42.9%) complete implant removal was possible during initial wound debridement. In five patients (35.7%) implants were replaced within the operation for defect closure. Hence, these operations were performed interdisciplinary with the orthopedic or neuro-surgeon. In three patients (21.4%), implant material had to be retained. These patients showed severe local wound infections, which required multiple revision surgeries including subsequent debridements and skin grafting. In 85.7% (n=12) pathogens were isolated in initial microbiological samples (33.3%: Staphylococcus aureus, E. coli, Enterococcae; 25%: Corynebacteria and Candida; 16.7%: Pseudomonas aeruginosa, Klebsiellae, Proteus). In two patients (14.3%) osteomyelitis was histologically proven. Consecutively, initial calculated antibiotic treatment was adapted according to resistograms.
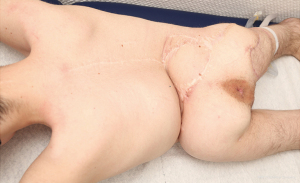
Mean time of flap surgery was 213 minutes (range, 77 to 480 minutes). Fifteen perforator-based flaps (53.6%) and 11 non-perforator (39.3%, conventional randomized rotational flaps), 1 pedicled muscle and 1 free latissimus dorsi flap were used. In 9 patients (64.3%) different flaps had to be combined in a single-staged procedure due to large wound sizes. Therefore, between 1 and up to 4 different flaps were transferred (mean: 2 flaps). In extensive wounds when more than two different flaps were used for defects closure (3 patients, 21.4%) additional skin grafts were necessary to cover flap donor sites. In three patients, local flaps were partially de-epithelialized and buried (2× perforator flaps, 1× conventional flap). In all cases, sufficient soft tissue reconstruction was achieved at patient discharge. No re-admission was necessary except from one case due to a new ischial defect after 29 months. In that patient, on admission the back still showed complete and stable wound closure as well (Figure 3). Hence, no late onset wound infections were detected. On average, 2.4 operations (ranging from 1 to 8) were performed to achieve complete closure of the defect. Mean hospital stay was prolonged with 35 days (range, 13 up to 66 days). No complete flap loss was detected. Subgroup analysis [only perforator flaps (“perf”; n=9), only randomized flaps (“nperf”; n=5), combination of both flap entities (“comb-p/n”; n=12)] demonstrate differences as followed (“perf” vs. “nperf” vs. “comb-p/n”): revision surgeries averaged 1.6 vs. 0 vs. 2.25. Differences between groups concerning revision surgeries were not significant (perf vs. nperf, P=0.412; nperf vs. comb-p/n, P=0.72 and perf vs. comb p/n, P=0.12). However, due to limited sample sizes statistical analysis may be underpowered. Defects in “nperf” were considerably smaller when compared to both other groups and surgery took less time in “nperf” (surgery time: 191.2 vs. 109 vs. 291.5 minutes); In 6 patients (42.9%) complications [impaired wound healing due to partial flap malperfusion (n=1), postoperative hematoma (n=1) or wound infections (n=4)] needed subsequent revision surgery. Out of these four cases of postoperative wound infection, in three cases implant material was not removed nor exchanged within soft tissue reconstruction surgery. In two cases (14.3%) immediate treatment was required. In one case a large hematoma was detected shortly after the operation, which significantly impeded flap perfusion and was drained successfully during revision surgery. Intra-operatively, another patient suddenly showed severe hemodynamic instability due to a pneumothorax during spinal implant exchange and was treated successfully with thoracic drainage.
Discussion
Wound healing problems after spinal surgery may lead to implant exposure, bacterial contamination and spinal infection. The need of contaminated implant removal is a feared complication due to the development of osseous non-union or delayed instability with neurological complications (13). Basically, the need of implant removal seems to be less likely if implant exposure is avoided and anti-microbiological and surgical treatment is initiated immediately (11,13-16). In consequence, to prevent implant exposure prophylactic soft tissue augmentation within spinal surgery in selected cases is an option. Thereby, local physiological conditions are restored immediately in terms of good vascularized tissue may improve wound healing and clear local bacteria load. In our patients’ cohort, only cases with prolonged exposure of spinal hardware were evaluated. Therefore, in the majority of cases implant removal or implant exchange was necessary after multi-disciplinary evaluation. In consequence, persisting wound infections and higher postoperative complication rates were likely. Soft tissue reconstruction in such cases is highly challenging and certain aspects should be considered preoperatively. In accordance to others, our data show that most patients with exposed spinal hardware present with multiple comorbidities and are in a significantly reduced general condition (13,17). Patients after spine surgery due to meningomyelocele, traumatic injuries or extended oncologic resections generally have long histories of surgeries as well as hospitalization. In the present study the majority underwent multiple previous surgeries and subsequently suffered from extended scars or extended radiation fields that significantly impede options for defect reconstruction. In these patients anatomical and geometrical variations may influence decision making for defect reconstruction. Severe contractures or considerable scoliosis may additionally impair classic perforator flaps in terms of flap design and flap sizes. Therefore, different techniques like uni- or bilateral perforator flaps, advancement or rotational flaps and split skin grafting of the donor sites are combined quite frequently (18). Local muscle flaps, foremost paraspinous muscle advancements are well described to cover central back defects (16,19-21). However, in the presented case series, these local advancement flaps have already been utilized during previous surgeries in an attempt to close the defects with local tissue. In contrast, others claim adipo-cutaneous or myocutaneous perforator flaps to be favorable in soft tissue reconstruction of spinal wounds (22). The authors agree with de Weerd et al., that perforator flaps may be advantageous concerning defect geometry due to more versatile flap positioning and flap-inset (22). In the majority of our cases, we covered the spine or implant material with perforator flaps due to its good vascularization and excellent inset characteristics. Flap design enables wound margins to be placed away from pressure sore predicted locations or directly above implant material, making wound dehiscence theoretically less likely (23). In contrast, in bilateral VY-shaped flaps or opposed rotational flaps compromised wound margins frequently have to be placed centrally over the spine or the spinal hardware for geometrical reasons, leading to an increased risk of wound brake down. Within perforator surgery, propeller flaps have some major advantages compared to other options for defect closure. Due to flap rotation uncompromised tissue is shifted to the defect with better capacities for wound healing. Furthermore, subcutaneous tissue on the lateral lower part of the trunk is thicker than medially and therefore subcutaneous augmentation is possible whenever propeller flaps are used. In consequence, even deep wound cavities can be filled in entirely after partial de-epithelialization and spinal hardware can be covered with plenty of vital tissue. In perforator flap harvest only limited undermining of the surrounding tissue is necessary which preserve circumferential tissues for subsequent “back-up” reconstructive options if needed. Since skin grafting is more likely for donor site closure in classical rotation flaps, perforator or propeller flaps may be advantageous in terms of primary donor site closure. Even if there are convincing arguments choosing propeller flaps for closure of dorsal soft tissue defects in our patients only one third of perforator flaps were propeller flaps. This is due to the fact that although propeller flaps may allow a decent flap inset perforator dissection and concomitant flap design may be difficult in these patients. Spinal deformities, radiation fields or multiple scarring make propeller flap design highly demanding and sometimes not feasible. Therefore, different reconstructive approaches should be considered as alternatives throughout the entire surgical procedure. This leads to a flexible operative approach that requires surgeon experience and confidence with the technique. A learning curve should be expected even in trained surgeons. Thereby, duplex ultrasound dramatically increases reliability of preoperative perforator mapping (24).Nevertheless, if the perforator is inapplicable for a propeller flap surgery flap design may be converted towards classical rotational or advancement flaps. In these cases, we always try to save already dissected perforators to maximize blood perfusion in crucial parts of conventional rotation flaps. These perforator-based advancement or rotation flaps were used quite frequently in our patients. Recently, intra-operative indocyanine green (ICG)-angiography helps to sufficiently objectify arterial and venous drainage of the flap in perforator surgery (25,26).
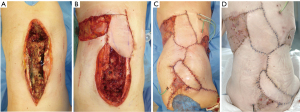
Due to large defects soft tissue reconstruction using only one flap including primary closure of the donor site in our patients was only exceptionally possible. In 64.3% of our patients combined reconstructive techniques with up to four different flaps were used to achieve wound closure (Figure 4).In consequence an individually adapted approach for soft tissue reconstruction in revisionary surgery represents the main defiance of such defects. Thereby, different plastic reconstructive techniques using perforator flaps, advancement or rotational flaps or even free flaps may be combined. Furthermore, a differentiated flap inset using several techniques such as propeller flap design, partially buried local flaps or combined skin graft transplantation of donor sites may be evaluated to achieve sustainable wound closure. Thereby, the authors used the following treatment algorithm for decision-making in these complex wounds (Figure 5). Foremost in large, multilevel central defects, therefore we frequently combined paraspinous perforator flaps centrally with classic non-perforator flaps or skin transplants laterally. Using perforator flaps in the first place, we try to shift the defect away from the complex prevertebral region to less demanding areas like the lateral trunk. In these regions, classic rotation or advancement flaps or skin transplants may be adequate to gain sufficient coverage without significantly prolonged surgery time. Thereby, a vast variety of different flap types may be combined due to defect size, location and geometry (Figure 6). In patients with very thin adipo-cutaneous tissue it may be advantageous to harvest myocutaneous perforator flaps in order to gain more soft tissue coverage and to improve cavity-filling capacities. Whenever donor site closure is not possible primarily due to impaired wound margins or significant tension, we decidedly encourage using additional flaps or skin grafts in the same operation to avoid impairment of flap perfusion or predictable wound dehiscence. Since we progressively used more than one flap for defect coverage, we only observed one case (perforator-based advancement flap) of distal partial flap loss (10%). Distal flap malperfusion is minimized in our clinical practice since we use ICG angiography intra-operatively as a standard. When perforator flaps were not possible due to inadequate perforating vessels classic flap options were evaluated to cover midline defects as well. In these cases, frequently more than one flap was necessary due to limited flap sizes and prior skin incisions. To avoid large soft tissue mobilization and wound margins placed pre-vertebrally free flap reconstruction should be evaluated as well. However, since suitable recipient vessels are rare in these cases, we only used free flap reconstruction once. Furthermore, the combination of av-loop and free flap surgery remains an option but should be evaluated carefully (27).Therefore, in our experience free flap reconstruction is only recommended in selected patients, whereas in the majority of cases a combination of local flaps may lead to reliable soft tissue reconstruction.
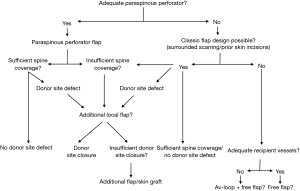
To date no concrete reconstructive treatment algorithm is present for these rare and heterogeneous cases (17). Classic approaches for defect coverage of the spine describe flap choice dependent on spine levels, such as upper, middle in lower third. In our experiences this does not really facilitate decision-making in flap choice in these patients. The absolute location of the wound does not properly indicate the real defiance of the reconstructive approach. In our opinion absolute defect size, previous surgery, the primary cause or implemented irradiation fields do have higher impact in flap choice and the entire reconstructive approach. Furthermore, severe circumferential scarring or entirely undermined wound margins may represent a more realistic scenario of the reconstructive challenges. Therefore, knowledge of wound biology and the geometry of wound size and cause are mandatory to find the individual and adjusted reconstructive approach for this comorbid patient cohort. Neither the anatomic location nor the absolute wound size may lead to a distinct reconstructive approach by itself. Furthermore, large defects do not automatically require more than one flap type. For example, in our cohort one large defect (300 cm2) was covered with only one latissimus free flap, which was used due to severe surrounding scars, which precluded any local flaps. However, free flap reconstruction in these comorbid patients maintains the risk of insufficient recipient vessels and postoperative perfusion control is highly demanding in terms of complex patient positioning. Thereby, the presented treatment algorithm may help in decision-making.
In this study a relevant rate of complications was predictably detected. Interestingly, those complications seem to occur independent from the technique chosen for defect closure. However, patient numbers are too small to detect significant differences. Furthermore, the evaluated group of patients is heterogeneous due to rare and selected cases. Thereby, a variety of different indications for previous spinal surgeries lead to complex wounds. However, the complexity of deep defects surrounded by large and indurated scars in the central back following spinal surgery is common in all cases and evaluation is reasonable. The majority of minor complications like wound-dehiscence or hematoma occurred within the first week post-operatively. Such complications are not completely preventable. More importantly, based on our findings prolonged wound infections seem to be more likely whenever implant material was not removed or replaced within flap surgery although antibiotic therapy and subsequent debridements were applied (21.4%). However, in this subset of patients implant removal was not possible due to incomplete osseous fusion and to minimize the risk of postoperative complications such as paralysis. Thereby an increased risk of wound healing problems and limited revision surgery due to prolonged wound secretion was accepted and distinctively discusses with the patient preoperatively.
Conclusions
In conclusion, based on our experiences, perforator-based flaps represent a very useful and potent option for defect closure in patients with prolonged exposure of spinal hardware, foremost when combined with other reconstructive techniques, if indicated properly. Thereby, within a multi-disciplinary approach exposed implant material exchange should be evaluated accurately. Soft tissue reconstruction of large, multilevel back wounds with exposed implants is complex and technically demanding not only due to perforator preparation. Foremost, a distinctive and individual treatment plan for soft tissue reconstruction is necessary. Thereby, a combination of different flaps is frequently mandatory to primarily address central back defects as well as large donor sites in these highly complex patients. The suggested treatment algorithm may help for intraoperative decision-making. A gold standard for treatment does—and most probably—will not exist soon because of this complex and heterogeneous patient cohort.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jss-20-688
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jss-20-688
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jss-20-688). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present analysis was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the governmental regional ethics committee (2019-14589). Due to the retrospective design of the present analysis written informed consent was not necessarily obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ishii M, Iwasaki M, Ohwada T, et al. Postoperative deep surgical-site infection after instrumented spinal surgery: a multicenter study. Global Spine J 2013;3:95-102. [Crossref] [PubMed]
- Manstein ME, Manstein CH, Manstein G. Paraspinous muscle flaps. Ann Plast Surg 1998;40:458-62. [Crossref] [PubMed]
- Singh K, Samartzis D, Heller JG, et al. The management of complex soft-tissue defects after spinal instrumentation. J Bone Joint Surg Br 2006;88:8-15. [Crossref] [PubMed]
- Dumanian GA, Ondra SL, Liu J, et al. Muscle flap salvage of spine wounds with soft tissue defects or infection. Spine (Phila Pa 1976) 2003;28:1203-11. [Crossref] [PubMed]
- Hallock GG. Reconstruction of posterior trunk defects. Semin Plast Surg 2011;25:78-85. [Crossref] [PubMed]
- Meiners T, Flieger R, Jungclaus M. Use of the reverse latissimus muscle flap for closure of complex back wounds in patients with spinal cord injury. Spine (Phila Pa 1976) 2003;28:1893-8. [Crossref] [PubMed]
- Basterzi Y, Tenekeci G. Dorsal Intercostal Artery Perforator Propeller Flaps: A Reliable Option in Reconstruction of Large Meningomyelocele Defects. Ann Plast Surg 2016;76:434-7. [Crossref] [PubMed]
- Brunetti B, Tenna S, Aveta A, et al. Posterior trunk reconstruction with the dorsal intercostal artery perforator based flap: Clinical experience on 20 consecutive oncological cases. Microsurgery 2016;36:546-51. [Crossref] [PubMed]
- Minabe T, Harii K. Dorsal intercostal artery perforator flap: anatomical study and clinical applications. Plast Reconstr Surg 2007;120:681-9. [Crossref] [PubMed]
- Kneser U, Beier JP, Schmitz M, et al. Zonal perfusion patterns in pedicled free-style perforator flaps. J Plast Reconstr Aesthet Surg 2014;67:e9-17. [Crossref] [PubMed]
- Maruo K, Berven SH. Outcome and treatment of postoperative spine surgical site infections: predictors of treatment success and failure. J Orthop Sci 2014;19:398-404. [Crossref] [PubMed]
- Labler L, Keel M, Trentz O, et al. Wound conditioning by vacuum assisted closure (V.A.C.) in postoperative infections after dorsal spine surgery. Eur Spine J 2006;15:1388-96. [Crossref] [PubMed]
- Chen SH, Lee CH, Huang KC, et al. Postoperative wound infection after posterior spinal instrumentation: analysis of long-term treatment outcomes. Eur Spine J 2015;24:561-70. [Crossref] [PubMed]
- Dipaola CP, Saravanja DD, Boriani L, et al. Postoperative infection treatment score for the spine (PITSS): construction and validation of a predictive model to define need for single versus multiple irrigation and debridement for spinal surgical site infection. Spine J 2012;12:218-30. [Crossref] [PubMed]
- Sayama C, Vadivelu S, Livingston A, et al. Soft-tissue defects after spinal instrumentation in 5 children: risk factors, management strategies, and outcomes. J Neurosurg Pediatr 2014;14:644-53. [Crossref] [PubMed]
- Hultman CS, Jones GE, Losken A, et al. Salvage of infected spinal hardware with paraspinous muscle flaps: anatomic considerations with clinical correlation. Ann Plast Surg 2006;57:521-8. [Crossref] [PubMed]
- Mericli AF, Largo RD, Garvey PB, et al. Immediate Reconstruction of Complex Spinal Wounds Is Associated with Increased Hardware Retention and Fewer Wound-related Complications: A Systematic Review and Meta-analysis. Plast Reconstr Surg Glob Open 2019;7:e2076 [Crossref] [PubMed]
- Park SW, Oh TS, Eom JS, et al. Freestyle multiple propeller flap reconstruction (jigsaw puzzle approach) for complicated back defects. J Reconstr Microsurg 2015;31:261-7. [Crossref] [PubMed]
- Cohen LE, Fullerton N, Mundy LR, et al. Optimizing Successful Outcomes in Complex Spine Reconstruction Using Local Muscle Flaps. Plast Reconstr Surg 2016;137:295-301. [Crossref] [PubMed]
- Mericli AF, Mirzabeigi MN, Moore JH Jr, et al. Reconstruction of complex posterior cervical spine wounds using the paraspinous muscle flap. Plast Reconstr Surg 2011;128:148-53. [Crossref] [PubMed]
- Mericli AF, Tarola NA, Moore JH Jr, et al. Paraspinous muscle flap reconstruction of complex midline back wounds: Risk factors and postreconstruction complications. Ann Plast Surg 2010;65:219-24. [Crossref] [PubMed]
- de Weerd L, Solberg TK, Weum S. Closure of Complex Posterior Midline Defects After Spinal Surgery With Sensate Midline-based Perforator Flaps and the Long-term Results. Spine (Phila Pa 1976) 2015;40:E1233-8. [Crossref] [PubMed]
- Schmidt VJ, Horch RE, Dragu A, et al. Myocutaneous propeller flap based on the superior gluteal artery (SGA) for closure of large lumbosacral meningomyelocoele defects: a case report. J Plast Reconstr Aesthet Surg 2012;65:521-4. [Crossref] [PubMed]
- Gunnarsson GL, Tei T, Thomsen JB. Color Doppler Ultrasonography-Targeted Perforator Mapping and Angiosome-Based Flap Reconstruction. Ann Plast Surg 2016;77:464-8. [Crossref] [PubMed]
- Jakubietz RG, Schmidt K, Bernuth S, et al. Evaluation of the Intraoperative Blood Flow of Pedicled Perforator Flaps Using Indocyanine Green-fluorescence Angiography. Plast Reconstr Surg Glob Open 2019;7:e2462 [Crossref] [PubMed]
- Bigdeli AK, Gazyakan E, Schmidt VJ, et al. Indocyanine Green Fluorescence for Free-Flap Perfusion Imaging Revisited: Advanced Decision Making by Virtual Perfusion Reality in Visionsense Fusion Imaging Angiography. Surg Innov 2016;23:249-60. [Crossref] [PubMed]
- Henn D, Wähmann MST, Horsch M, et al. One-Stage versus Two-Stage Arteriovenous Loop Reconstructions: An Experience on 103 Cases from a Single Center. Plast Reconstr Surg 2019;143:912-24. [Crossref] [PubMed]




