
Comparing hyperlordotic and standard lordotic cages for achieving segmental lumbar lordosis during transforaminal lumbar interbody fusion in adult spinal deformity surgery
Introduction
Patients with adult spinal deformity require sagittal balance correction, including lumbar lordosis, generally through spinal fusion procedures (1). Transforaminal lumbar fusion (TLIF) provides one such posterior interbody approach to fuse the spine. The use of cages and technology for the procedure continue to evolve, offering new modalities to control the degree of lordosis produced in the lumbar spine. In particular, hyperlordotic cages have been introduced to offer surgeons greater control of the lordosis that they can achieve, without having to rely overly on osteotomies and other, more invasive, measures (2).
Interbody cages can vary drastically in the amount of lordosis with which they are designed. Despite the variability in the lordosis designed into cages, reviews of the lumbar lordosis produced, as measured radiographically, have been conflicting. Takahashi et al. found that there was no difference in the amount of lordosis created between 3° lordosis cages and non-lordotic cages in the posterior lumbar interbody fusion (PLIF) procedure (3). Diedrich et al. examined cages with 4° lordosis, hypothesizing that these cages could create significantly more segmental lumbar lordosis (SLL) than non-lordotic cages in the PLIF procedure (4). Here, too, there was no significant difference between the wedged and non-wedged cages. Govindasamy et al. reached a more radical conclusion, stating that standalone bone graft was just as efficacious as interbody cages in lumbar interbody fusion procedures (5).
In contrast, Sembrano et al. indicates that lordotic cages provide significant increases in segmental lordosis in the lateral lumbar interbody fusion procedure (6). Cho et al. examined the use of 4° and 8° lordotic cages in degenerative lumbar disease. While both types of cages allowed for adequate fusion, the 4° lordotic cages resulted in a loss of lumbar lordosis compared to the 8° cages (7). In a previous study, Cho et al. examined 81 patients undergoing PLIF with a 4° cage, observing that those cages are unable to maintain lumbar lordosis, though disc height was maintained (8). Hong et al. (9) expanded upon these studies, by examining the results of 4° and 8° cages in comparison to 15° cages. They found that 15° cages perform best in maintaining lumbar lordosis; these cages increased lumbar lordosis from 31.1° preoperatively to 42.9° postoperatively and 36.4° at final follow-up.
Many institutions have shifted toward hyperlordotic cages, suggesting that they offer an advantage over cages with less lordosis in producing SLL. Few studies, however, directly compare the effect of interbody cages with different degrees of lordosis in producing SLL in the TLIF procedure and as such is the objective of the present analysis.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jss-21-15).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Columbia University (NO.: AAAQ9223) and individual consent for this retrospective analysis was waived. Following Institutional Review Board approval, 38 consecutive adult spine deformity patients undergoing corrective spinal surgery involving a TLIF procedure by a single surgeon (LGL) between 2017 and 2018 were enrolled. Study data were collected and managed using a patient-protected database (10). Patients receiving one and two-level TLIF procedures were included; those receiving TLIFs at more than two levels were excluded from this study. Additionally, patients who had more than one type of cage placed were excluded. After exclusion criteria, patients’ charts were then reviewed using our institution’s electronic health record and Picture Archiving and Communications System (PACS).
Patients were grouped into those receiving either hyperlordotic cages or standard lordotic cages. Patients with “hyperlordotic” cages received a titanium interbody cage with 20° of lordosis designed into the cage. Those receiving the “standard lordotic” cage received a titanium cage with 6° of lordosis designed into the cage. Erect films were used to measure SLL, both preoperatively and postoperatively. Postoperative standing radiographs were typically obtained during the same admission as the surgery, closer to the day of discharge. SLL was measured, using a validated PACS viewer, in the standard fashion, from the superior endplate of the vertebra above the cage to the inferior endplate of the vertebra below the cage (11). As depicted in Figure 1, a one-level TLIF from L4–L5 required measuring the Cobb angle from the superior endplate of L4 to the inferior endplate of L5.

We then determined the change in SLL achieved in the immediate postoperative period relative to the corresponding preoperative values, using the erect preoperative and postoperative films. Statistical analysis, using paired t-tests, compared these changes among individual patients (in one-level or two-level TLIF). The changes in lordosis were then compared between the standard lordotic and hyperlordotic cages using the independent t-test. Statistical analysis was done using SAS (Gary, NC) and significance was set at P<0.05.
Results
There were 23 patients who had a one-level TLIF and 15 patients who had a two-level TLIF. Among those with a one-level TLIF, 11 had standard lordotic cages; and 12 had hyperlordotic cages. Among those with a two-level TLIF, seven had standard lordotic cages (14 total cages); and eight had hyperlordotic cages (16 total cages). The mean age for those receiving less-lordotic cages was 52.0±16.7 years old, and the mean age for those receiving hyper-lordotic cages was 50.8±14.2 years old. In the group receiving less-lordotic cages, 72% (13 of 18) were female; in the group with hyper-lordotic cages, 90% (18 of 20) were female. The vast majority of cages placed were at L5/S1 (Figure 2). A Chi-square test of independence indicated that the distribution of cages by level did not vary significantly between less-lordotic and hyper-lordotic cages (P=0.42).
Postoperative erect films were obtained 6.8±4.7 days after surgery for those who received less-lordotic cages and 8.1±6.6 days after surgery for those who received hyper-lordotic cages. 14.6±4.6 levels had been fused among those who received less-lordotic cages, whereas 14±3.2 levels had been fused among those who received hyper-lordotic cages. Seventy-eight percent (14 of 18 patients) of those who received less-lordotic cages had undergone a previous fusion procedure. Seventy-five percent (15 of 20 patients) of those who received hyper-lordotic cages had undergone a previous fusion.
Among patients receiving a one-level TLIF, SLL changed from 22.6°±14.7° to 30.4°±9.2° on average after placement of a standard lordotic cage (P=0.024, Table 1). SLL changed from 21.2°±12.8° to 29.4°±9.7° on average after placement of a hyper-lordotic cages in the one-level TLIF group (P=0.020). While there was a significant change in SLL with both types of cages, hyperlordotic cages did not produce more postoperative SLL than standard lordotic cages (P=0.917).
Table 1
| TLIF procedure, cage design, and number of levels fused | Preop. SLL (°) | Postop. SLL (°) | 1-year SLL (°) | ΔSLL (°) |
P, ΔSLL |
P, ΔSLL (hyperlordotic |
|---|---|---|---|---|---|---|
| One-level TLIF (n=23) | ||||||
| Standard lordotic cages (n=11) | 22.62 | 30.38 | 27.19 | 7.76 | 0.024 | 0.917 |
| Hyperlordotic cages (n=12) | 21.21 | 29.42 | 29.90 | 8.21 | 0.020 | |
| Two-level TLIF (n=30) | ||||||
| Standard lordotic cages (n=14) | 29.34 | 43.24 | 42.33 | 13.90 | 0.032 | 0.389 |
| Hyperlordotic cages (n=16) | 37.29 | 46.11 | 40.92 | 8.83 | 0.023 |
TLIF, transforaminal lumbar interbody fusion; SLL, segmental lumbar lordosis.
Similarly, among the two-level TLIF group, there were significant increases in SLL with both standard lordotic and hyperlordotic cages. Standard lordotic cages produced an increase in SLL from 29.3°±14.9° to 43.2°±6.9° (P=0.032). Hyper-lordotic cages resulted in an increase in SLL from 37.3°±17.9° to 46.1°±14.4° on average (P=0.023). However, just as we found in the one-level TLIF cohort, the changes in SLL were not significantly different between the two types of cages (P=0.389). Moreover, though the change in segmental lordosis was greater when performing TLIFs at two levels instead of one (11.2° vs. 8.0°), it did not reach statistical significance (P=0.355) (Figure 3).
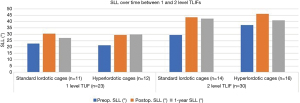
When examining patients with the lowest 25% of preoperative SLLs and those with the highest 25% of SLLs, some differences become apparent (Table 2). For both standard lordotic cages and hyperlordotic cages, there is a significant increase in SLL postoperatively among the lowest quartile of preoperative SLLs (P=0.035, P=0.012). In contrast, among those patients with preoperative SLLs that are in the upper quartile, there is a significant change in SLL neither in patients with standard lordotic nor in patients with hyperlordotic cages (P=0.686, P=0.415). Among the patients with a significant increase in preop SLL, namely those whose preoperative SLLs were in the lowest quartile, there was no significant difference in the change in SLL (preop vs. postop) between cage types (P=0.998).
Table 2
| Cage type | Lowest 25% preop. SLL | Highest 25% preop. SLL | P, ΔSLL (standard |
|||||
|---|---|---|---|---|---|---|---|---|
| Preop. | Postop. | P | Preop. | Postop. | P | |||
| Standard lordotic cages | 5.72 | 25.38 | 0.035 | 41.24 | 42.52 | 0.686 | 0.998 | |
| Hyperlordotic cages | 6.72 | 26.36 | 0.012 | 46.44 | 47.92 | 0.415 | ||
SLL, segmental lumbar lordosis.
One-year follow-up data was available for 75% of patients who had received hyper-lordotic cages and 89% of patients who had received standard lordotic cages (Figure 4). At 1-year follow-up, the standard lordotic cages produced, on average, 35.5°±9.7° SLL (preop. 25.2°±14.8°). The hyperlordotic cages produced, on average, 33.6°±9.1° SLL (preop. 27.6°±16.7°) at 1 year (Table 3). On average, the SLL did not change significantly (P=0.55).
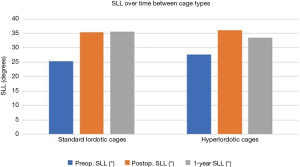
Table 3
| Cage design | Preop. SLL (°) | Postop. SLL (°) | 1-year SLL (°) | P (postop. |
|---|---|---|---|---|
| Standard lordotic cages | 25.23 | 35.38 | 35.51 | 0.501 |
| Hyperlordotic cages | 27.64 | 36.10 | 33.57 | 0.781 |
SLL, segmental lumbar lordosis.
Discussion
Hyperlordotic cages have been advertised as being able to increase the degree of lumbar lordosis in spinal surgery correcting deformity. While the hyperlordotic cages do, on average, significantly increase a given patient’s SLL, they do not appear to perform better than standard lordotic cages in our analysis. Importantly, these procedures were performed with the same surgeon using both types of cages, which corrects for inter-operator variability.
While it is evident in our results that both cage types can significantly increase SLL, it should be noted cages are not always placed with the goal of increasing SLL. This point is underscored when comparing the changes in SLL among the patients with the highest and lowest preoperative SLLs (Table 2). Those patients in the upper quartile of preoperative SLLs did not necessarily require a substantial increase in SLL; accordingly, there was not a significant increase in SLL postoperatively among these patients. Conversely, those patients with the lowest 25% of preoperative SLLs experienced a significant increase in SLL. That the same cage can produce a larger increase in SLL in one group of patients than in another highlights the importance of surgical technique in deformity correction. Moreover, neither cage type was superior to the other when examining patients whose preoperative SLLs were in the lowest quartile.
The use of a single surgeon’s operative cases is a potential limitation to this study, as a particular surgeon may favor techniques that diminish the relative effect of cages. Conversely, analyzing one surgeon’s results may allow one to isolate the effect of the cages, which might otherwise have been confounded by variations in operative technique. Additionally, measuring SLL in relatively early postoperative images may be problematic as the final sagittal balance may not be established for several months until after the procedure. Salem et al. examined segmental and total lumbar lordosis (TLL) in 84 patients, both in the early postoperative period and at 6 months postoperatively. They found that both SLL and TLL changed over time (12). However, utilizing postoperative images obtained a few days postoperatively standardized our comparisons of lordosis that might otherwise be confounded by varying lengths of time prior to follow-up.
Notably, our standard lordotic cage had 6° of slope designed into the cage. Therefore, it remains possible that a cage with 0° lordosis may be inferior to the cages in this study in producing SLL. Moreover, we were unable to control for the variability from case to case as the surgeon may use his clinical judgment to determine if further operative intervention is required to generate additional lordosis, complicating comparisons of hyperlordotic and standard lordotic cages. Thus, surgeon technique is likely at least as important as the degree of lordosis in interbody cages. Additionally, the anteroposterior location of the cage within a given disk space was not specifically assessed in this study and may have an impact on the amount of lordosis achieved. However, this is likely mitigated by using a single surgeon’s cases. Finally, there may be a ceiling effect on the amount of lordosis that can be achieved with increasing lordosis of the interbody implant.
These results support the findings in Hong et al.’s study, which demonstrate improvement in SLL postoperatively with 4°, 8°, and 15° interbody cages (9). However, Hong’s study suggests that the lordosis generated by the 8° and 15° cages is greater than that generated by the 4° cages, which differs from our results. Our data does not demonstrate any difference in the amount of lordosis produced between the different cages. Their study assessed 67 patients with 15° cages, 49 patients with 8° cages, and 65 patients with 4° cages. Notably, in this study, cages could not be uniformly used in patients; narrow disc spaces were only given 4° and 8° cages, which may affect the observed outcomes. Our results suggest that, while they can significantly increase postoperative SLL, hyperlordotic cages do not do so more effectively than standard lordotic cages.
Conclusions
Although it is theorized that hyperlordotic cages would increase SLL during open TLIF procedures more than standard lordotic cages, our data failed to demonstrate that. As our study examined cases performed by a single surgeon immediately before and after adoption of these lordotic cages, it is likely that surgical technique is of equal or greater importance in improving SLL than the amount of lordosis designed into interbody cages.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jss-21-15
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jss-21-15
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jss-21-15). ZMS reports personal fees from Medtronic, outside the submitted work. LGL reports personal fees from Medtronic, grants and personal fees from DePuy-Synthes Spine, personal fees from K2M, non-financial support from Broadwater, non-financial support from Seattle Science Foundation, grants and non-financial support from Scoliosis Research Society, non-financial support from Stryker Spine, non-financial support from The Spinal Research Foundation, grants from EOS, grants from Setting Scoliosis Straight Foundation, personal fees from Fox Rothschild, LLC, personal fees from Quality Medical Publishing, other from Evans Family Donation, other from Fox Family Foundation, grants and non-financial support from AOSpine, outside the submitted work. LGL serves as an unpaid editorial board member of Journal of Spine Surgery from Oct 2019 to Oct 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Columbia University (No.: AAAQ9223) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schwab F, Lafage V, Patel A, et al. Sagittal plane considerations and the pelvis in the adult patient. Spine (Phila Pa 1976) 2009;34:1828-33. [Crossref] [PubMed]
- Anand N, Cohen RB, Cohen J, et al. The Influence of Lordotic cages on creating Sagittal Balance in the CMIS treatment of Adult Spinal Deformity. Int J Spine Surg 2017;11:23. [Crossref] [PubMed]
- Takahashi H, Suguro T, Yokoyama Y, et al. Effect of cage geometry on sagittal alignment after posterior lumbar interbody fusion for degenerative disc disease. J Orthop Surg (Hong Kong) 2010;18:139-42. [Crossref] [PubMed]
- Diedrich O, Perlick L, Schmitt O, et al. Radiographic spinal profile changes induced by cage design after posterior lumbar interbody fusion preliminary report of a study with wedged implants. Spine (Phila Pa 1976) 2001;26:E274-80. [Crossref] [PubMed]
- Govindasamy R, Solomon P, Sugumar D, et al. Is the Cage an Additional Hardware in Lumbar Interbody Fusion for Low Grade Spondylolisthesis? A Prospective Study. J Clin Diagn Res 2017;11:RC05-8. [Crossref] [PubMed]
- Sembrano JN, Horazdovsky RD, Sharma AK, et al. Do Lordotic Cages Provide Better Segmental Lordosis Versus Nonlordotic Cages in Lateral Lumbar Interbody Fusion (LLIF)? Clin Spine Surg 2017;30:E338-43. [Crossref] [PubMed]
- Cho KJ, Kim YT. Transforaminal Lumbar Interbody Fusion with Local Bone Graft in Degenerative Lumbar Spinal Disease: A Comparison of 4° Lordotic Angle Cage and 8° Cage. Spine J 2014;14:S151. [Crossref]
- Cho KJ, Kim YT, Park SR, et al. Restoration of Lumbar Lordosis After Posterior Lumbar Interbody Fusion with 4 Degree Cage in Degenerative Spinal Disease. J Korean Soc Spine Surg 2013;20:51-7. [Crossref]
- Hong TH, Cho KJ, Kim YT, et al. Does Lordotic Angle of Cage Determine Lumbar Lordosis in Lumbar Interbody Fusion? Spine (Phila Pa 1976) 2017;42:E775-80. [Crossref] [PubMed]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [Crossref] [PubMed]
- Kim SB, Jeon TS, Heo YM, et al. Radiographic results of single level transforaminal lumbar interbody fusion in degenerative lumbar spine disease: focusing on changes of segmental lordosis in fusion segment. Clin Orthop Surg 2009;1:207-13. [Crossref] [PubMed]
- Salem KMI, Eranki AP, Paquette S, et al. Do intraoperative radiographs predict final lumbar sagittal alignment following single-level transforaminal lumbar interbody fusion? J Neurosurg Spine 2018;28:486-91. [Crossref] [PubMed]



