
Minimally invasive transforaminal lumbar interbody fusion with expandable articulating interbody spacers significantly improves radiographic outcomes compared to static interbody spacers
Introduction
There are numerous approaches to a lumbar interbody fusion in the surgical treatment of degenerative lumbar diseases, yet each has inherent advantages and disadvantages to be considered. The transforaminal lumbar interbody fusion (TLIF) technique allows for positive surgical outcomes while addressing the limitations of other approaches, such as the risk of vascular injury in anterior lumbar interbody fusion and the extent of neural retraction required in a posterior lumbar interbody fusion (1,2). Minimally invasive surgery for TLIF (MI TLIF), while necessitating a learning curve, has been associated with decreased operative time, reduced blood loss, shorter hospital stays, improved cost-effectiveness, and decreased pain with its minimized retraction of posterior muscles (3-16). The MI TLIF approach is susceptible to difficulties in sagittal alignment correction and disc height restoration (1,8,17-19). Maintenance of spinopelvic parameters is associated with improved surgical and clinical outcomes (20,21), indicating the significance of improving these standards in an MI TLIF approach.
The use of static spacers in an MI TLIF requires excessive trialing, endplate preparation, and overdistraction, which may lead to an increased risk of subsidence and, therefore, compromised biomechanical stability (22,23). These challenges have commonly been attributed to a small spacer footprint, placement of the spacer in the middle of the disc where bone is less robust, and excessive trialing required before insertion (24-27). To help eliminate these challenges, an articulating banana-shaped lordotic expandable spacer, used with the MI TLIF approach, was specifically designed to articulate horizontally along the anterior column and then expand to the desired height. There may be benefits to using this spacer instead of a static TLIF spacer, such as preventing overdistraction during insertion, added stability, and increased lordosis. The purpose of this study is to compare the radiographic outcomes of patients treated with an articulating expandable spacer with lordosis to those treated with a static spacer in a MI TLIF procedure.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jss-20-630).
Methods
Patient population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This was a multi-site, multi-surgeon, Institutional Review Board-exempt, retrospective clinical study from a prospectively collected database. This article was a multi-site retrospective study of patient records that did not require consent from human subjects. No patient consent was sought or received. It included 48 patients with a diagnosis of degenerative disc disease (DDD) at one level from L2 to S1 with or without Grade 1 spondylolisthesis. All patients underwent MI TLIF using either an articulating expandable interbody spacer (ALTERA®, Globus Medical, Inc., Audubon, PA, USA) or a static interbody spacer, with supplemental posterior pedicle screw and rod fixation (Figure 1). Radiographic images were assessed for sagittal alignment parameters.

Outcome measures
Radiographic parameters including disc height, neuroforaminal height, intervertebral angle, segmental lordosis, and lumbar lordosis were assessed preoperatively (Figure 2) and at 6 weeks, 3 and 6 months, and final follow-up postoperatively. Disc heights were measured from the superior to inferior endplate at the anterior, middle, and posterior portions of the disc space in the lateral plane. Neuroforaminal height was measured as the distance from the inferior pedicle wall of the level above to the superior pedicle wall of the level below. Intervertebral angle was measured as the angle between the superior and inferior endplates. Segmental lordosis was defined as the angle between the inferior endplate of the caudal vertebral body and the superior endplate of the cephalad vertebral body. Lumbar lordosis was measured from the endplate of S1 to the superior endplate of L1.
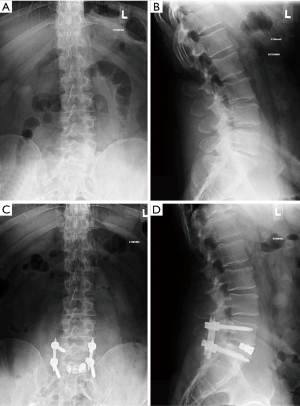
Surgical technique
Under general anesthesia, patients were positioned in the prone position using a Jackson table. After the operative level was identified under fluoroscopy, a percutaneous incision was made 4 cm lateral of midline (or wider, depending on patient anatomy) and in line with the disc. A total facetectomy and hemilaminectomy were performed.
Discectomy was performed preserving the annulus, and endplates were prepared. Trialing was performed, and the appropriate-sized implant was selected and filled with autogenous bone graft in the cases where the articulating expandable spacer was used, and a mixture of bone marrow aspirate, demineralized cortical fiber matrix (FiberOS™; Organogenesis, Canton, MA, USA) and bioactive amniotic suspension (HCT/P) (Nucel®, Organogenesis, Canton, MA, USA) was used in the static spacer cases. The articulating expandable interbody device was inserted into the disc space in the collapsed state until it reached the anterior anulus. The implant was then articulated to its final position, slightly recessed from the anterior wall of the vertebral body. The spacer was then expanded until it reached desired height and was confirmed on intraoperative fluoroscopy. The implant was expanded with caution, using tactile feel and the torque limiting driver, to avoid over distraction or damage to the endplate. Additional graft material was backfilled into the expanded spacer and surrounding disc space. For static spacers, multiple trialing and endplate preparation was necessary to fit a fixed-sized interbody spacer. The implant was then impacted into the disc space at an oblique angle. Finally, pedicle screws and rods were implanted for posterior supplemental fixation in both groups.
Interbody spacers
The expandable articulating interbody spacer used in this study is manufactured from titanium alloy. The device is inserted at a contracted height and expanded in situ once correctly positioned within the intervertebral space, offering continuous expansion for optimal endplate-to-endplate contact. The static interbody spacer is manufactured from radiolucent polymer with titanium alloy or tantalum markers.
Statistical analysis
Statistical analysis was performed with SPSS® v20.0.0 software for Windows (IBM Corp., Armonk, NY, USA). Descriptive statistics were recorded as mean and standard deviation, or frequency and percentage, where applicable. Paired and independent sampled t-tests were used to calculate differences in radiographic outcomes. Statistical significance was set at P<0.05.
Results
Patient demographic data
The average age of the 48 patients enrolled in this study was 54.1±10.6 years, and 50.0% of the patients were female. Of the operative levels, 20/48 (41.7%) were at L4–L5 and 24/48 (50.0%) were at L5–S1.
Twenty-seven patients underwent TLIF surgery and were implanted with the articulating expandable spacer. The average age was 55.7±9.5 years, and 55.6% were female. The average follow-up for the articulating expandable spacer group was 9.1 months. The majority of the operative levels in this group were L5–S1 (51.9%) and L4–L5 (37.0%).
Twenty-one patients were implanted with a static spacer after undergoing a TLIF. The average age of these patients was 52.1±11.9 years, and 42.9% were female. The average follow-up was 16 months. Of the operative levels in the static spacer group, 47.6% were at L4–L5, and 47.6% were at L5–S1. Baseline characteristics are presented in Table 1.
Table 1
| Parameter | Articulating expandable | Static |
|---|---|---|
| Number of patients | 27 | 21 |
| Gender, n (%) | ||
| Female | 12 (44.4) | 12 (57.1) |
| Male | 15 (55.6) | 9 (42.9) |
| Age, mean ± SD, (range) | 55.7±9.5 [34–70] | 52.1±11.9 [29–76] |
| Average follow-up | 9.1 months | 16.0 months |
| Levels treated, n (%) | ||
| L3–L4 | 3 (11.1) | 0 (0.0) |
| L4–L5 | 10 (37.0) | 10 (47.6) |
| L5–S1 | 14 (51.9) | 10 (47.6) |
| L6–S1 | 0 (0.0) | 1 (4.8) |
SD, standard deviation.
Radiographic outcomes
Raw values for each time point are presented in Table 2, and the average improvements from baseline are presented in Table 3.
Table 2
| Parameter | Device | Baseline | 6 weeks | 3 months | 6 months | Final follow-up |
|---|---|---|---|---|---|---|
| ADH (mm) | Expandable | 10.1±3.1 | 14.5±1.7* | 14.5±1.7* | 14.7±1.9* | 14.7±2.0* |
| Static | 7.5±2.9 | 9.0±2.3* | 9.1±2.1* | 9.0±2.3* | 8.4±1.7 | |
| PDH (mm) | Expandable | 5.7±2.0 | 8.7±2.5* | 8.0±2.2* | 8.0±2.3* | 8.2±2.2* |
| Static | 4.4±1.8 | 6.2±1.2* | 6.0±1.1* | 5.9±1.1* | 5.1±1.1 | |
| NFH (mm) | Expandable | 18.8±4.7 | 21.1±4.6* | 21.2±4.8* | 20.9±4.4* | 22.2±7.8* |
| Static | 17.4±3.9 | 17.7±5.0 | 17.5±4.9 | 18.0±3.8 | 16.7±4.0 | |
| IVA (°) | Expandable | 6.9±4.2 | 8.7±5.3 | 9.9±5.2* | 9.8±5.4* | 9.9±5.2* |
| Static | 4.7±3.3 | 5.0±3.4 | 5.0±2.8 | 5.1±3.2 | 5.0±2.6 | |
| Segmental lordosis (°) | Expandable | 19.0±7.0 | 20.5±8.1 | 22.3±7.1* | 20.2±8.2 | 20.6±8.5 |
| Static | 16.9±6.1 | 16.4±6.4 | 16.4±6.1 | 16.5±5.1 | 15.7±5.6 | |
| Lumbar lordosis (°) | Expandable | 53.5±12.1 | 54.2±11.3 | 59.7±10.9* | 57.1±11.7* | 55.8±13.8* |
| Static | 47.5±11.3 | 46.5±10.2 | 48.5±10.1 | 49.0±8.8 | 48.5±9.7 |
*, P<0.05 compared to baseline. Mean ± SD. ADH, anterior disc height; PDH, posterior disc height; NFH, neuroforaminal height; IVA, Intervertebral Angle; SD, standard deviation.
Table 3
| Parameter | Device | 6 weeks | 3 months | 6 months | Final follow-up |
|---|---|---|---|---|---|
| ADH (mm) | Expandable | 4.6±3.2* | 4.4±3.6* | 4.9±3.5* | 4.6±3.4* |
| Static | 2.0±2.5 | 1.6±2.3 | 1.5±2.6 | 0.8±2.3 | |
| PDH (mm) | Expandable | 3.0±2.1 | 2.3±2.4 | 2.5±1.9* | 2.5±2.1* |
| Static | 2.1±2.0 | 1.8±2.0 | 1.3±1.8 | 0.8±1.9 | |
| NFH (mm) | Expandable | 2.4±3.8 | 2.5±4.5 | 1.8±3.5 | 3.3±7.7* |
| Static | 0.5±2.6 | 0.2±2.6 | 0.2±2.9 | −0.7±3.0 | |
| IVA (°) | Expandable | 1.7±5.0 | 2.6±4.6* | 3.3±4.7* | 3.3±4.9* |
| Static | 0.3±3.2 | 0.1±2.8 | 0.5±3.4 | 0.2±2.7 | |
| Segmental lordosis (°) | Expandable | 1.7±7.0 | 3.3±5.6* | 1.4±6.0 | 1.5±5.7 |
| Static | 0.0±3.0 | −0.5±3.5 | −0.3±4.3 | −1.2±4.0 | |
| Lumbar lordosis (°) | Expandable | 1.8±6.3 | 5.2±6.1* | 4.4±5.3* | 2.7±6.7 |
| Static | 0.5±7.1 | 0.8±5.6 | 0.4±5.9 | 1.0±5.9 |
*, P<0.05 compared to static. Mean ± SD. ADH, anterior disc height; PDH, posterior disc height; NFH, neuroforaminal height; IVA, Intervertebral Angle; SD, standard deviation.
Within-group comparisons
In the articulating expandable spacer group, anterior disc height significantly increased from baseline by an average of 44% (4.6±3.2 mm), 43% (4.4±3.6 mm), 45% (4.9±3.5 mm), and 45% (4.6±3.4 mm) at 6 weeks, 3 and 6 months, and final follow-up, respectively (P<0.05). In the static spacer group, anterior disc height significantly increased by an average of 20% (2.0±2.5 mm), 21% (1.6±2.3 mm), and 19% (1.5±2.6 mm) from baseline at 6 weeks, 3 and 6 months, respectively (P<0.05). Anterior disc height also increased by an average of 11% (0.8±2.3 mm) from baseline at the final follow-up; however, improvement was not significant.
Average posterior disc height significantly increased from baseline in the articulating expandable spacer group by 52% (3.0±2.1 mm), 40% (2.3±2.4 mm), 41% (2.5±1.9 mm), and 43% (2.5±2.1 mm) at 6 weeks, 3 and 6 months, and final follow-up, respectively (P<0.05). Posterior disc height significantly increased from baseline in the static spacer group by an average of 42% (2.1±2.0 mm), 37% (1.8±2.0 mm), and 34% (1.3±1.8 mm) at 6 weeks, 3 and 6 months, respectively (P<0.05). The 18% (0.8±1.9 mm) increase from baseline at final follow-up was not significant.
In the articulating expandable spacer group, mean neuroforaminal height significantly increased at 6 weeks, 3 and 6 months, and final follow-up by 12% (2.4±3.8 mm), 13% (2.5±4.5 mm), 11% (1.8±3.5 mm), and 18% (3.3±7.7 mm), respectively (P<0.05). Average neuroforaminal height increased by 1.4% (0.5±2.6 mm), 0.3% (0.2±2.6 mm), and 3.3% (0.2±2.9 mm) at 6 weeks, 3 and 6 months, respectively, in the static spacer group, but improvement was not significant. Neuroforaminal height decreased from baseline at final follow-up by a mean of 4.1% (−0.7±3.0 mm) in the static spacer group.
Intervertebral angle significantly increased from baseline by averages of 43% (2.6±4.6°), 41% (3.3±4.7°), and 43% (3.3±4.9°) at 3 and 6 months, and final follow-up, respectively, in the articulating expandable spacer group (P<0.05). In the static spacer group, intervertebral angle increased by averages of 5% (0.3±3.2°), 5% (0.1±2.8°), 8% (0.5±3.4°), and 5% (0.2±2.7°) from baseline at 6 weeks, 3 and 6 months, and final follow-up, respectively, yet improvement was not significant.
Average segmental lordosis increased from baseline in the articulating expandable spacer group by 8% (1.7±7.0°), 17% (3.3±5.6°), 6% (1.4±6.0°), and 8% (1.5±5.7°) at 6 weeks, 3 and 6 months, and final follow-up, respectively, with improvement at 3 months being significant (P<0.05). Segmental lordosis decreased from baseline in the static spacer group by averages of 3% (0.0±3.0°), 3% (−0.5±3.5°), 2% (−0.3±4.3°), and 7% (−1.2±4.0°) at 6 weeks, 3 and 6 months, and final follow-up, respectively.
In the articulating expandable spacer group, mean lumbar lordosis significantly increased at 3 and 6 months, and final follow-up by 12% (5.2±6.1°), 7% (4.4±5.3°), and 4% (2.7±6.7°), respectively (P<0.05). Average lumbar lordosis in the static spacer group increased by 1.2% (0.5±7.1°), 2.1% (0.8±5.6°), 3.2% (0.4±5.9°), and 2.1% (1.0±5.9°) at 6 weeks, 3 and 6 months, and final follow-up respectively, but improvement was not significant.
Between-group comparisons
The articulating expandable group resulted in a significantly greater increase in anterior disc height compared to the static group at 6 weeks, 3 and 6 months, and final follow-up by averages of 2.6 mm (79%), 2.8 mm (92%), 3.4 mm (105%), and 3.8 mm (139%), respectively (P<0.05). Mean increases in posterior disc height (Figure 3) were significantly greater in the expandable group compared to the static group by 1.2 mm (65%) and 1.7 mm (104%) at 6 months and final follow-up, respectively (P<0.05). The mean improvement in neuroforaminal height (Figure 3) from baseline to final follow-up was significantly greater in the articulating expandable spacer group than in the static spacer group by 4.0 mm (P<0.05).
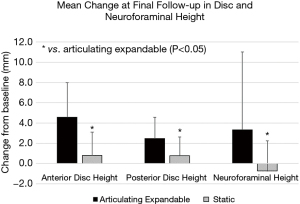
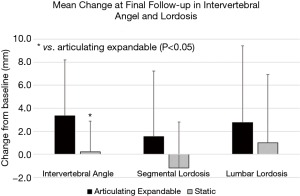
Articulating expandable spacers resulted in a significantly greater increase in intervertebral angle compared to the static group at 3 and 6 months, and final follow-up by averages of 2.5°, 2.8°, and 3.1°, respectively (P<0.05). The mean increase in segmental lordosis (Figure 4) was significantly greater in the expandable group compared to the static group by 3.8° at 3 months (P<0.05). The mean improvement in lumbar lordosis (Figure 4) from baseline to 3 and 6 months was significantly greater in the articulating expandable spacer group than in the static spacer group by 4.4° and 4.0°, respectively (P<0.05).
Discussion
New technology such as an articulating expandable MI TLIF spacer requires clinical studies to evaluate its safety, efficacy, and durability by collecting clinical and radiographic outcomes. Sagittal alignment restoration is associated with improved clinical outcomes and overall surgical success (20,21). The present study showed that an articulating expandable spacer significantly improves disc height, neuroforaminal height, and intervertebral angle compared to a static spacer at final follow-up.
To the authors’ knowledge, there are only two previous articles that discuss the radiographic outcomes of an articulating expandable MI TLIF spacer. The first article is a similar comparative evaluation to the current study by Hawasli et al. (28). These researchers found similar results to the present study when comparing an articulating expandable device to static spacers. A significant 33% greater disc height and 30% greater segmental lordosis were achieved at final follow-up in the articulating expandable spacer group compared to the static spacer group. A 15% greater neuroforaminal height recorded in patients with the articulating expandable spacer was also reported. However, in contrast to the current study, there was not a significant improvement in overall lumbar lordosis in the articulating expandable spacer group.
The second article is a non-comparative 2018 observational study by Massie et al. (29), in which investigators evaluated 44 patients with the same articulating expandable device used in the present study at an average final follow-up of 18 months. They found significant improvement from baseline in posterior disc height and segmental lordosis by averages of 3.2 mm and 3.1°, respectively. These results are consistent with the current study; however, the current study also found significant improvements in anterior disc height and neuroforaminal height that were not measured in the analysis by Massie.
The possible reasons that articulating expandable MI TLIF spacers resulted in improved sagittal alignment compared to static spacers is twofold. First, expandable spacers are inserted at a contracted height and expanded in situ, permitting greater disc height restoration compared to hammering in a static spacer. Multiple studies have reported on the benefits of expandable technology when used with a MI TLIF procedure, including less force during impaction leading to less subsidence and an increase in disc height restoration (30-33). Second, the device articulates to a final position on the anterior apophyseal ring, the strongest part of the interbody space, which allows for increased biomechanical stability (27,34). The center of the vertebral body, where MI TLIF static spacers are typically placed, is the weakest portion of the body, making this area susceptible to subsidence (24-27). Subsidence rates from the use of static spacers in a MI TLIF procedure can range anywhere from 0% to 52% (23,35-37). Long-term studies are needed to compare the risk of subsidence in patients instrumented with traditional static TLIF spacers placed in the middle of the vertebral body to the risk associated with articulating expandable TLIF spacers placed on the anterior apophyseal ring of the vertebral body. Additionally, anterior placement of an MI TLIF spacer on the apophyseal ring leads to increased lordosis (28,29). There is a significant relationship between the anterior placement of TLIF spacers and induction of lordosis (38).
There are some notable limitations in this study. Data were collected from a small sample size of patients with a short follow-up. The final follow-up differed between groups. Two surgeons with different surgical techniques were involved in this study, making direct comparisons difficult. Specifically, two different bone graft materials were used for each group. One could argue that a more osteoconductive/osteoinductive mixture was used in the static spacer group and thus gave an advantage to the static spacer group. Additionally, radiographic measurements were conducted by different observers, but were verified by an orthopaedic surgeon. Further studies should focus on larger patient cohorts with clinical outcomes and a longer follow-up period to assess the durability of an articulating expandable device.
Conclusions
The current study showed that articulating expandable TLIF spacers are superior to static TLIF spacers in restoring disc height, neuroforaminal height, intervertebral angle, and segmental lordosis. Future studies are needed to evaluate articulating expandable spacers’ effects on clinical outcomes, but radiographic results are promising.
Acknowledgments
Funding: This work was supported by Globus Medical, Inc.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jss-20-630
Peer Review File: Available at https://dx.doi.org/10.21037/jss-20-630
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jss-20-630). Dr. AJR reports personal fees from Globus Medical, Inc., during the conduct of the study; personal fees from Orthofix, personal fees from Surgentec, outside the submitted work; Dr. SAS reports personal fees from Globus Medical, Inc., during the conduct of the study; personal fees from RTI Surgical, personal fees from Organogenesis, from Arthrex, outside the submitted work; Mr. TS reports personal fees from Globus Medical, Inc., during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work (including full data access, integrity of the data and the accuracy of the data analysis) in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This was a multi-site, multi-surgeon, Institutional Review Board-exempt, retrospective clinical study from a prospectively collected database. This article was a multi-site retrospective study of patient records that did not require consent from human subjects. No patient consent was sought or received.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teng I, Han J, Phan K, et al. A meta-analysis comparing ALIF, PLIF, TLIF and LLIF. J Clin Neurosci 2017;44:11-7. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Ge DH, Stekas ND, Varlotta CG, et al. Comparative Analysis of Two Transforaminal Lumbar Interbody Fusion Techniques: Open TLIF Versus Wiltse MIS TLIF. Spine (Phila Pa 1976) 2019;44:E555-60. [Crossref] [PubMed]
- Li A, Li X, Zhong Y. Is minimally invasive superior than open transforaminal lumbar interbody fusion for single-level degenerative lumbar diseases: a meta-analysis. J Orthop Surg Res 2018;13:241. [Crossref] [PubMed]
- Parker SL, Adogwa O, Bydon A, et al. Cost-effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis associated low-back and leg pain over two years. World Neurosurg 2012;78:178-84. [Crossref] [PubMed]
- Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg 2014;82:230-8. [Crossref] [PubMed]
- Patel AA, Zfass-Mendez M, Lebwohl NH, et al. Minimally Invasive Versus Open Lumbar Fusion: A Comparison of Blood Loss, Surgical Complications, and Hospital Course. Iowa Orthop J 2015;35:130-4. [PubMed]
- Price JP, Dawson JM, Schwender JD, et al. Clinical and Radiologic Comparison of Minimally Invasive Surgery With Traditional Open Transforaminal Lumbar Interbody Fusion: A Review of 452 Patients From a Single Center. Clin Spine Surg 2018;31:E121-6. [Crossref] [PubMed]
- Qin R, Liu B, Zhou P, et al. Minimally Invasive Versus Traditional Open Transforaminal Lumbar Interbody Fusion for the Treatment of Single-Level Spondylolisthesis Grades 1 and 2: A Systematic Review and Meta-Analysis. World Neurosurg 2019;122:180-9. [Crossref] [PubMed]
- Sharif S, Afsar A. Learning Curve and Minimally Invasive Spine Surgery. World Neurosurg 2018;119:472-8. [Crossref] [PubMed]
- Silva PS, Pereira P, Monteiro P, et al. Learning curve and complications of minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus 2013;35:E7 [Crossref] [PubMed]
- Singh K, Nandyala SV, Marquez-Lara A, et al. A perioperative cost analysis comparing single-level minimally invasive and open transforaminal lumbar interbody fusion. Spine J 2014;14:1694-701. [Crossref] [PubMed]
- Sulaiman WA, Singh M. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis grades 1-2: patient-reported clinical outcomes and cost-utility analysis. Ochsner J 2014;14:32-7. [PubMed]
- Tan JH, Liu G, Ng R, et al. Is MIS-TLIF superior to open TLIF in obese patients?: A systematic review and meta-analysis. Eur Spine J 2018;27:1877-86. [Crossref] [PubMed]
- Xie L, Wu WJ, Liang Y. Comparison between Minimally Invasive Transforaminal Lumbar Interbody Fusion and Conventional Open Transforaminal Lumbar Interbody Fusion: An Updated Meta-analysis. Chin Med J (Engl) 2016;129:1969-86. [Crossref] [PubMed]
- Zhao J, Zhang S, Li X, et al. Comparison of Minimally Invasive and Open Transforaminal Lumbar Interbody Fusion for Lumbar Disc Herniation: A Retrospective Cohort Study. Med Sci Monit 2018;24:8693-8. [Crossref] [PubMed]
- Lim JK, Kim SM. Radiographic Results of Minimally Invasive (MIS) Lumbar Interbody Fusion (LIF) Compared with Conventional Lumbar Interbody Fusion. Korean J Spine 2013;10:65-71. [Crossref] [PubMed]
- Carlson BB, Saville P, Dowdell J, et al. Restoration of lumbar lordosis after minimally invasive transforaminal lumbar interbody fusion: a systematic review. Spine J 2019;19:951-8. [Crossref] [PubMed]
- Ahlquist S, Park HY, Gatto J, et al. Does approach matter? A comparative radiographic analysis of spinopelvic parameters in single-level lumbar fusion. Spine J 2018;18:1999-2008. [Crossref] [PubMed]
- Glassman SD, Berven S, Bridwell K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 2005;30:682-8. [Crossref] [PubMed]
- Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024-9. [Crossref] [PubMed]
- Torretti J, Harris JA, Bucklen BS, et al. In Vitro Biomechanical and Fluoroscopic Study of a Continuously Expandable Interbody Spacer Concerning Its Role in Insertion Force and Segmental Kinematics. Asian Spine J 2018;12:601-10. [Crossref] [PubMed]
- Lee N, Kim KN, Yi S, et al. Comparison of Outcomes of Anterior, Posterior, and Transforaminal Lumbar Interbody Fusion Surgery at a Single Lumbar Level with Degenerative Spinal Disease. World Neurosurg 2017;101:216-26. [Crossref] [PubMed]
- Oxland TR, Grant JP, Dvorak MF, et al. Effects of endplate removal on the structural properties of the lower lumbar vertebral bodies. Spine (Phila Pa 1976) 2003;28:771-7. [Crossref] [PubMed]
- Tan JS, Bailey CS, Dvorak MF, et al. Interbody device shape and size are important to strengthen the vertebra-implant interface. Spine (Phila Pa 1976) 2005;30:638-44. [Crossref] [PubMed]
- Lowe TG, Hashim S, Wilson LA, et al. A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine (Phila Pa 1976) 2004;29:2389-94. [Crossref] [PubMed]
- Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976) 2001;26:889-96. [Crossref] [PubMed]
- Hawasli AH, Khalifeh JM, Chatrath A, et al. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg Focus 2017;43:E10 [Crossref] [PubMed]
- Massie LW, Zakaria HM, Schultz LR, et al. Assessment of radiographic and clinical outcomes of an articulating expandable interbody cage in minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis. Neurosurg Focus 2018;44:E8 [Crossref] [PubMed]
- Mica MC, Voronov LI, Carandang G, et al. Biomechanics of an Expandable Lumbar Interbody Fusion Cage Deployed Through Transforaminal Approach. Int J Spine Surg 2018;12:520-7. [Crossref] [PubMed]
- Boktor JG, Pockett RD, Verghese N. The expandable transforaminal lumbar interbody fusion - Two years follow-up. J Craniovertebr Junction Spine 2018;9:50-5. [PubMed]
- Kim CW, Doerr TM, Luna IY, et al. Minimally Invasive Transforaminal Lumbar Interbody Fusion Using Expandable Technology: A Clinical and Radiographic Analysis of 50 Patients. World Neurosurg 2016;90:228-35. [Crossref] [PubMed]
- Tassemeier T, Haversath M, Jäger M. Transforaminal lumbar interbody fusion with expandable cages: Radiological and clinical results of banana-shaped and straight implants. J Craniovertebr Junction Spine 2018;9:196-201. [Crossref] [PubMed]
- Polly DW Jr, Klemme WR, Cunningham BW, et al. The biomechanical significance of anterior column support in a simulated single-level spinal fusion. J Spinal Disord 2000;13:58-62. [Crossref] [PubMed]
- Heinz von der Hoeh N, Villa T, Galbusera F, et al. Analysis of a Unilateral Bridging Cage for Lumbar Interbody Fusion: 2-Year Clinical Results and Fusion Rate with a Focus on Subsidence. World Neurosurg 2018;116:e308-14. [Crossref] [PubMed]
- Lin GX, Quillo-Olvera J, Jo HJ, et al. Minimally Invasive Transforaminal Lumbar Interbody Fusion: A Comparison Study Based on End Plate Subsidence and Cystic Change in Individuals Older and Younger than 65 Years. World Neurosurg 2017;106:174-84. [Crossref] [PubMed]
- Pereira C, Santos Silva P, Cunha M, et al. How Does Minimally Invasive Transforaminal Lumbar Interbody Fusion Influence Lumbar Radiologic Parameters? World Neurosurg 2018;116:e895-902. [Crossref] [PubMed]
- Kwon BK, Berta S, Daffner SD, et al. Radiographic analysis of transforaminal lumbar interbody fusion for the treatment of adult isthmic spondylolisthesis. J Spinal Disord Tech 2003;16:469-76. [Crossref] [PubMed]


